Host Age Prediction from Fecal Microbiota Composition in Male C57BL/6J Mice
- PMID: 35674443
- PMCID: PMC9241839
- DOI: 10.1128/spectrum.00735-22
Host Age Prediction from Fecal Microbiota Composition in Male C57BL/6J Mice
Abstract
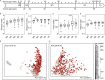
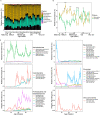
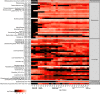
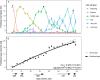
The lifelong relationship between microorganisms and hosts has a profound impact on the overall health and physiology of the holobiont. Microbiome composition throughout the life span of a host remains largely understudied. Here, the fecal microbiota of conventionally raised C57BL/6J male mice was characterized throughout almost the entire adult life span, from "maturing" (9 weeks) until "very old" (112 weeks) age. Our results suggest that microbiota changes occur throughout life but are more pronounced in maturing to middle-age mice than in mice later in life. Phylum-level analysis indicates a shift of the Bacteroidota-to-Firmicutes ratio in favor of Firmicutes in old and very old mice. More Firmicutes amplicon sequence variants (ASVs) were transient with varying successional patterns than Bacteroidota ASVs, which varied primarily during maturation. Microbiota configurations from five defined life phases were used as training sets in a Bayesian model, which effectively enabled the prediction of host age. These results suggest that age-associated compositional differences may have considerable implications for the interpretation and comparability of animal model-based microbiome studies. The sensitivity of the age prediction to dietary perturbations was tested by applying this approach to two age-matched groups of C57BL/6J mice that were fed either a standard or western diet. The predicted age for the western diet-fed animals was on average 27 ± 11 (mean ± standard deviation) weeks older than that of standard diet-fed animals. This indicates that the fecal microbiota-based predicted age may be influenced not only by the host age and physiology but also potentially by other factors such as diet. IMPORTANCE The gut microbiome of a host changes with age. Cross-sectional studies demonstrate that microbiota of different age groups are distinct but do not demonstrate the temporal change that a longitudinal study is able to show. Here, we performed a longitudinal study of adult mice for over 2 years. We identified life stages where compositional changes were more dynamic and showed temporal changes for the more abundant species. Using a Bayesian model, we could reliably predict the life stages of the mice. Application of the same training set to mice fed different dietary regimens revealed that life-stage age predictions were possible for mice fed the same diet but less so for mice fed different diets. This study sheds light on the temporal changes that occur within the gut microbiota of laboratory mice over their life span and may inform researchers on the appropriate mouse age for their research.
Keywords: aging; gut microbiota; mouse microbiota.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Rausch P, Basic M, Batra A, Bischoff SC, Blaut M, Clavel T, Glasner J, Gopalakrishnan S, Grassl GA, Gunther C, Haller D, Hirose M, Ibrahim S, Loh G, Mattner J, Nagel S, Pabst O, Schmidt F, Siegmund B, Strowig T, Volynets V, Wirtz S, Zeissig S, Zeissig Y, Bleich A, Baines JF. 2016. Analysis of factors contributing to variation in the C57BL/6J fecal microbiota across German animal facilities. Int J Med Microbiol 306:343–355. doi: 10.1016/j.ijmm.2016.03.004. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

