Attenuated brain white matter functional network interactions in Parkinson's disease
- PMID: 35674466
- PMCID: PMC9491278
- DOI: 10.1002/hbm.25973
Attenuated brain white matter functional network interactions in Parkinson's disease
Abstract
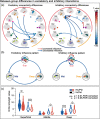
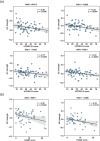
Parkinson's disease (PD) is a neurodegenerative disorder characterized by extensive structural abnormalities in cortical and subcortical brain areas. However, an association between changes in the functional networks in brain white matter (BWM) and Parkinson's symptoms remains unclear. With confirming evidence that resting-state functional magnetic resonance imaging (rs-fMRI) of BWM signals can effectively describe neuronal activity, this study investigated the interactions among BWM functional networks in PD relative to healthy controls (HC). Sixty-eight patients with PD and sixty-three HC underwent rs-fMRI. Twelve BWM functional networks were identified by K-means clustering algorithm, which were further classified as deep, middle, and superficial layers. Network-level interactions were examined via coefficient Granger causality analysis. Compared with the HC, the patients with PD displayed significantly weaker functional interaction strength within the BWM networks, particularly excitatory influences from the superficial to deep networks. The patients also showed significantly weaker inhibitory influences from the deep to superficial networks. Additionally, the sum of the absolutely positive/negative regression coefficients of the tri-layered networks in the patients was lower relative to HC (p < .05, corrected for false discovery rate). Moreover, we found the functional interactions involving the deep BWM networks negatively correlated with part III of the Unified Parkinson's Disease Rating Scales and Hamilton Depression Scales. Taken together, we demonstrated attenuated BWM interactions in PD and these abnormalities were associated with clinical motor and nonmotor symptoms. These findings may aid understanding of the neuropathology of PD and its progression throughout the nervous system from the perspective of BWM function.
Keywords: Granger causality analysis; Parkinson's disease; interactions; motor and nonmotor symptoms; white matter functional networks.
© 2022 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Braak, H. , Del Tredici, K. , Rüb, U. , De Vos, R. A. , Steur, E. N. J. , & Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of Aging, 24(2), 197–211. - PubMed
-
- Chen, G. , Hamilton, J. P. , Thomason, M. E. , Gotlib, I. H. , Saad, Z. S. , & Cox, R. W. (2009). Granger causality via vector auto‐regression tuned for fMRI data analysis. Proceedings of the International Society for Magnetic Resonance in Medicine, 17, 1718.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

