The immunology of Parkinson's disease
- PMID: 35674826
- PMCID: PMC9519672
- DOI: 10.1007/s00281-022-00947-3
The immunology of Parkinson's disease
Abstract
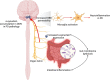
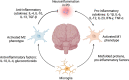
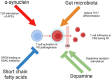
Parkinson's disease (PD) is the second most common neurodegenerative disorder which affects 6.1 million people worldwide. The neuropathological hallmarks include the loss of dopaminergic neurons in the substantia nigra, the presence of Lewy bodies and Lewy neurites caused by α-synuclein aggregation, and neuroinflammation in the brain. The prodromal phase happens years before the onset of PD during which time many patients show gastro-intestinal symptoms. These symptoms are in support of Braak's theory and model where pathological α-synuclein propagates from the gut to the brain. Importantly, immune responses play a determinant role in the pathogenesis of Parkinson's disease. The innate immune responses triggered by microglia can cause neuronal death and disease progression. In addition, T cells infiltrate into the brains of PD patients and become involved in the adaptive immune responses. Interestingly, α-synuclein is associated with both innate and adaptive immune responses by directly interacting with microglia and T cells. Here, we give a detailed review of the immunobiology of Parkinson's disease, focusing on the role α-synuclein in the gut-brain axis hypothesis, the innate and adaptive immune responses involved in the disease, and current treatments.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

