Interim 2-Year Analysis from SERENA: A Real-World Study in Patients with Psoriatic Arthritis or Ankylosing Spondylitis Treated with Secukinumab
- PMID: 35674938
- PMCID: PMC9174439
- DOI: 10.1007/s40744-022-00460-x
Interim 2-Year Analysis from SERENA: A Real-World Study in Patients with Psoriatic Arthritis or Ankylosing Spondylitis Treated with Secukinumab
Abstract
Introduction: Sustained improvement of high degree in clinical outcomes have been demonstrated in phase 3 trials with secukinumab in both psoriatic arthritis (PsA) and ankylosing spondylitis (AS). The objective of the SERENA study was to evaluate the effectiveness, retention rates, and safety of secukinumab in patients with PsA and AS.
Methods: SERENA is an ongoing, longitudinal, real-world observational study involving patients with moderate-to-severe psoriasis, PsA, or AS. Patients had received at least 16 weeks of secukinumab treatment before recruitment to the study. Retention rate was defined as percentage of patients who continued secukinumab treatment over the course of study. Effectiveness of secukinumab in AS and PsA cohorts was assessed using descriptive statistics.
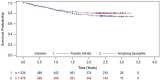
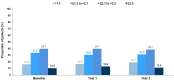
Results: The current interim analysis included 1004 patients with PsA or AS. Overall secukinumab retention rates at 2 years after enrolment were 74.9 and 78.9% in patients with PsA and AS, respectively. At baseline and at 2 years, swollen joint count [3.3 (5.8) vs. 2.9 (5.8)], tender joint count [6.3 (9.4) vs. 5.6 (7.2)] in patients with PsA and BASDAI scores [3.2 (2.3) vs. 2.9 (2.3)] in patients with AS, suggest sustained effectiveness for patients remaining on secukinumab for at least 2 years after enrolment. A total of 73 patients had treatment interruption; 78% of these patients reinitiated secukinumab without a loading dose. No new or unexpected safety signals were reported.
Conclusions: After more than 2 years since initiation, secukinumab demonstrated high retention rates and favorable safety profile as well as sustained effectiveness in patients who continued secukinumab treatment.
Keywords: Ankylosing spondylitis; Efficacy; Psoriatic arthritis; Retention; Secukinumab.
© 2022. The Author(s).
Figures
References
-
- Schreiber S, Colombel J-F, Feagan BG, et al. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann Rheum Dis. 2019;78(4):473–479. doi: 10.1136/annrheumdis-2018-214273. - DOI - PMC - PubMed
-
- Coates LC, Orbai AM, Azevedo VF, et al. Results of a global, patient-based survey assessing the impact of psoriatic arthritis discussed in the context of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire. Health Qual Life Outcomes. 2020;18(1):173. doi: 10.1186/s12955-020-01422-z. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous