Methylmalonic Acid Impairs Cell Respiration and Glutamate Uptake in C6 Rat Glioma Cells: Implications for Methylmalonic Acidemia
- PMID: 35674974
- PMCID: PMC11414442
- DOI: 10.1007/s10571-022-01236-1
Methylmalonic Acid Impairs Cell Respiration and Glutamate Uptake in C6 Rat Glioma Cells: Implications for Methylmalonic Acidemia
Abstract
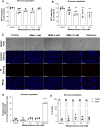
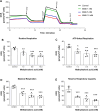
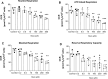
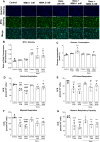
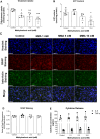
Methylmalonic acidemia is an organic acidemia caused by deficient activity of L-methylmalonyl-CoA mutase or its cofactor cyanocobalamin and it is biochemically characterized by an accumulation of methylmalonic acid (MMA) in tissue and body fluids of patients. The main clinical manifestations of this disease are neurological and observable symptoms during metabolic decompensation are encephalopathy, cerebral atrophy, coma, and seizures, which commonly appear in newborns. This study aimed to investigate the toxic effects of MMA in a glial cell line presenting astrocytic features. Astroglial C6 cells were exposed to MMA (0.1-10 mM) for 24 or 48 h and cell metabolic viability, glucose consumption, and oxygen consumption rate, as well as glutamate uptake and ATP content were analyzed. The possible preventive effects of bezafibrate were also evaluated. MMA significantly reduced cell metabolic viability after 48-h period and increased glucose consumption during the same period of incubation. Regarding the energy homeostasis, MMA significantly reduced respiratory parameters of cells after 48-h exposure, indicating that cell metabolism is compromised at resting and reserve capacity state, which might influence the cell capacity to meet energetic demands. Glutamate uptake and ATP content were also compromised after exposure to MMA, which can be influenced energy metabolism impairment, affecting the functionality of the astroglial cells. Our findings suggest that these effects could be involved in the pathophysiology of neurological dysfunction of this disease. Methylmalonic acid compromises mitochondrial functioning leading to reduced ATP production and reduces glutamate uptake by C6 astroglial cells.
Keywords: Cell dysfunction; Energy metabolism; Glutamate uptake; High-resolution respirometry; Methylmalonic academia; Organic acidemias.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
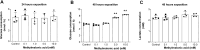
References
-
- Baumgartner MR, Horster F, Dionisi-Vici C, Haliloglu G, Karall D, Chapman KA, Huemer M, Hochuli M, Assoun M, Ballhausen D, Burlina A, Fowler B, Grunert SC, Grunewald S, Honzik T, Merinero B, Perez-Cerda C, Scholl-Burgi S, Skovby F, Wijburg F, MacDonald A, Martinelli D, Sass JO, Valayannopoulos V, Chakrapani A (2014) Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J Rare Dis 9:130. 10.1186/s13023-014-0130-8 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

