Rates of Response to Atogepant for Migraine Prophylaxis Among Adults: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 35675076
- PMCID: PMC9178435
- DOI: 10.1001/jamanetworkopen.2022.15499
Rates of Response to Atogepant for Migraine Prophylaxis Among Adults: A Secondary Analysis of a Randomized Clinical Trial
Abstract
Importance: Some patients with migraine, particularly those in primary care, require effective, well-tolerated, migraine-specific oral preventive treatments.
Objective: To examine the efficacy of atogepant, an oral, small-molecule, calcitonin gene-related peptide receptor antagonist, using 4 levels of mean monthly migraine-day (MMD) responder rates.
Design, setting, and participants: This secondary analysis of a phase 3, double-blind, placebo-controlled randomized clinical trial evaluated the efficacy and safety of atogepant for the preventive treatment of migraine from December 14, 2018, to June 19, 2020, in adults with 4 to 14 migraine-days per month at 128 sites in the US.
Interventions: Patients were administered 10 mg of atogepant (n = 222), 30 mg of atogepant (n = 230), 60 mg of atogepant (n = 235), or placebo (n = 223) once daily in a 1:1:1:1 ratio for 12 weeks.
Main outcomes and measures: These analyses evaluated treatment responder rates, defined as participants achieving 50% or greater (α-controlled, secondary end point) and 25% or greater, 75% or greater, and 100% (prespecified additional end points) reductions in mean MMDs during the 12-week blinded treatment period.
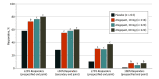
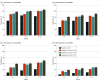
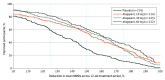
Results: Of 902 participants (mean [SD] age, 41.6 [12.3] years; 801 [88.8%] female; 752 [83.4%] White; 825 [91.5%] non-Hispanic), 873 were included in the modified intention-to-treat population (placebo, 214; 10 mg of atogepant, 214; 30 mg of atogepant, 223; and 60 mg of atogepant, 222). For the secondary end point, a 50% or greater reduction in the 12-week mean of MMDs was achieved by 119 of 214 participants (55.6%) treated with 10 mg of atogepant (odds ratio, 3.1; 95% CI, 2.1-4.6), 131 of 223 participants (58.7%) treated with 30 mg atogepant (odds ratio, 3.5; 95% CI, 2.4-5.3), 135 of 222 participants (60.8%) treated with 60 mg of atogepant (odds ratio, 3.8; 95% CI, 2.6-5.7), and 62 of 214 participants (29.0%) given placebo (P < .001). The numbers of participants who reported a 25% or greater reduction in the 12-week mean of MMDs were 157 of 214 (73.4%) for 10 mg of atogepant, 172 of 223 (77.1%) for 30 mg of atogepant, and 180 of 222 (81.1%) for 60 mg of atogepant vs 126 of 214 (58.9%) for placebo (P < .002). The numbers of participants who reported a 75% or greater reduction in mean MMDs were 65 of 214 (30.4%) for 10 mg of atogepant, 66 of 223 (29.6%) for 30 mg of atogepant, and 84 of 222 (37.8%) for 60 mg of atogepant compared with 23 of 214 (10.7%) for placebo (P < .001). The numbers of participants reporting 100% reduction in mean MMDs were 17 of 214 (7.9%) for 10 mg of atogepant (P = .004), 11 of 223 (4.9%) for 30 mg of atogepant (P = .02), and 17 of 222 (7.7%) for 60 mg of atogepant (P = .003) compared with 2 of 214 (0.9%) for placebo.
Conclusions and relevance: At all doses, atogepant was effective during the 12-week double-blind treatment period beginning in the first 4 weeks, as evidenced by significant reductions in mean MMDs at every responder threshold level. Higher atogepant doses appeared to produce the greatest responder rates, which can guide clinicians in individualizing starting doses.
Trial registration: ClinicalTrials.gov Identifier: NCT03777059.
Conflict of interest statement
Figures