Diastereoselectivity is in the Details: Minor Changes Yield Major Improvements to the Synthesis of Bedaquiline
- PMID: 35675114
- PMCID: PMC9545417
- DOI: 10.1002/chem.202201311
Diastereoselectivity is in the Details: Minor Changes Yield Major Improvements to the Synthesis of Bedaquiline
Abstract
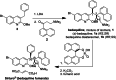
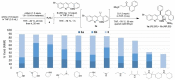
Bedaquiline is a crucial medicine in the global fight against tuberculosis, yet its high price places it out of reach for many patients. Herein, we describe improvements to the key industrial lithiation-addition sequence that enable a higher yielding and therefore more economical synthesis of bedaquiline. Prioritization of mechanistic understanding and multi-lab reproducibility led to optimized reaction conditions that feature an unusual base-salt pairing and afford a doubling of the yield of racemic bedaquiline. We anticipate that implementation of these improvements on manufacturing scale will be facile, thereby substantially increasing the accessibility of this essential medication.
Keywords: bedaquiline; continuous flow; diastereoselectivity; lithiation; nucleophilic addition.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Global tuberculosis report 2021. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/teams/global-tuberculosis-programme/tb-reports.
-
- Médecins Sans Frontières Issue Brief: DR-TB Drugs Under the Microscope, 6th edition. October, 2019.
-
- Calvert M. B., Furkert D. P., Cooper C. B., Brimble M. A., Bioorg. Med. Chem. Lett. 2020, 30, 127172. - PubMed
-
- Cox E., Laessig K., N. Engl. J. Med. 2014, 371, 689–691. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

