Staphylococcus aureus cell wall maintenance - the multifaceted roles of peptidoglycan hydrolases in bacterial growth, fitness, and virulence
- PMID: 35675307
- PMCID: PMC9616470
- DOI: 10.1093/femsre/fuac025
Staphylococcus aureus cell wall maintenance - the multifaceted roles of peptidoglycan hydrolases in bacterial growth, fitness, and virulence
Abstract
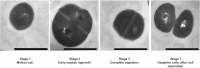
Staphylococcus aureus is an important human and livestock pathogen that is well-protected against environmental insults by a thick cell wall. Accordingly, the wall is a major target of present-day antimicrobial therapy. Unfortunately, S. aureus has mastered the art of antimicrobial resistance, as underscored by the global spread of methicillin-resistant S. aureus (MRSA). The major cell wall component is peptidoglycan. Importantly, the peptidoglycan network is not only vital for cell wall function, but it also represents a bacterial Achilles' heel. In particular, this network is continuously opened by no less than 18 different peptidoglycan hydrolases (PGHs) encoded by the S. aureus core genome, which facilitate bacterial growth and division. This focuses attention on the specific functions executed by these enzymes, their subcellular localization, their control at the transcriptional and post-transcriptional levels, their contributions to staphylococcal virulence and their overall importance in bacterial homeostasis. As highlighted in the present review, our understanding of the different aspects of PGH function in S. aureus has been substantially increased over recent years. This is important because it opens up new possibilities to exploit PGHs as innovative targets for next-generation antimicrobials, passive or active immunization strategies, or even to engineer them into effective antimicrobial agents.
Keywords: Staphylococcus aureus; antimicrobial susceptibility; cell wall; pathogenesis; peptidoglycan hydrolase; subcellular protein localization.
© The Author(s) 2022. Published by Oxford University Press on behalf of FEMS.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

