Chromatographic separation of active polymer-like worm mixtures by contour length and activity
- PMID: 35675403
- PMCID: PMC9177071
- DOI: 10.1126/sciadv.abj7918
Chromatographic separation of active polymer-like worm mixtures by contour length and activity
Abstract
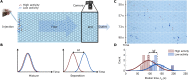
The convective transport rate of polymers through confined geometries depends on their size, allowing for size-based separation of polymer mixtures (chromatography). Here, we investigate whether mixtures of active polymers can be separated in a similar manner based on their activity. We use thin, living Tubifex tubifex worms as a model system for active polymers and study the transport of these worms by an imposed flow through a channel filled with a hexagonal pillar array. The transport rate through the channel depends strongly on the degree of activity, an effect that we assign to the different distribution of conformations sampled by the worms depending on their activity. Our results demonstrate a unique way to sort mixtures of active polymers based on their activity and provide a versatile and convenient experimental system to investigate the hydrodynamics of active polymers.
Figures




References
-
- Marchetti M. C., Joanny J. F., Ramaswamy S., Liverpool T. B., Prost J., Rao M., Simha R. A., Hydrodynamics of soft active matter. Phys. Rev. Lett. 85, 1143–1189 (2013).
-
- Salbreux G., Charras G., Paluch E., Actin cortex mechanics and cellular morphogenesis. Trends Cell Biol. 22, 536–545 (2012). - PubMed
LinkOut - more resources
Full Text Sources

