Glucose-sensing glucagon-like peptide-1 receptor neurons in the dorsomedial hypothalamus regulate glucose metabolism
- PMID: 35675406
- PMCID: PMC9177072
- DOI: 10.1126/sciadv.abn5345
Glucose-sensing glucagon-like peptide-1 receptor neurons in the dorsomedial hypothalamus regulate glucose metabolism
Abstract
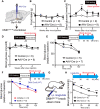
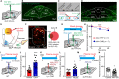
Glucagon-like peptide-1 (GLP-1) regulates energy homeostasis via activation of the GLP-1 receptors (GLP-1Rs) in the central nervous system. However, the mechanism by which the central GLP-1 signal controls blood glucose levels, especially in different nutrient states, remains unclear. Here, we defined a population of glucose-sensing GLP-1R neurons in the dorsomedial hypothalamic nucleus (DMH), by which endogenous GLP-1 decreases glucose levels via the cross-talk between the hypothalamus and pancreas. Specifically, we illustrated the sufficiency and necessity of DMHGLP-1R in glucose regulation. The activation of the DMHGLP-1R neurons is mediated by a cAMP-PKA-dependent inhibition of a delayed rectifier potassium current. We also dissected a descending control of DMHGLP-1R -dorsal motor nucleus of the vagus nerve (DMV)-pancreas activity that can regulate glucose levels by increasing insulin release. Thus, our results illustrate how central GLP-1 action in the DMH can induce a nutrient state-dependent reduction in blood glucose level.
Figures





References
-
- Bell G. I., Sanchez-Pescador R., Laybourn P. J., Najarian R. C., Exon duplication and divergence in the human preproglucagon gene. Nature 304, 368–371 (1983). - PubMed
-
- Drucker D. J., Glucagon and the glucagon-like peptides. Pancreas 5, 484–488 (1990). - PubMed
-
- Drucker D. J., Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 27, 740–756 (2018). - PubMed
-
- Müller T. D., Finan B., Bloom S. R., D’Alessio D., Drucker D. J., Flatt P. R., Fritsche A., Gribble F., Grill H. J., Habener J. F., Holst J. J., Langhans W., Meier J. J., Nauck M. A., Perez-Tilve D., Pocai A., Reimann F., Sandoval D. A., Schwartz T. W., Seeley R. J., Stemmer K., Tang-Christensen M., Woods S. C., Di Marchi R. D., Tschöp M. H., Glucagon-like peptide 1 (GLP-1). Mol. Metab. 30, 72–130 (2019). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

