Cav1.3 calcium channels are full-range linear amplifiers of firing frequencies in lateral DA SN neurons
- PMID: 35675413
- PMCID: PMC9177074
- DOI: 10.1126/sciadv.abm4560
Cav1.3 calcium channels are full-range linear amplifiers of firing frequencies in lateral DA SN neurons
Abstract
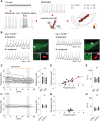
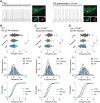
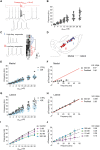
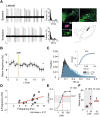
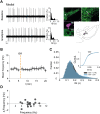
The low-threshold L-type calcium channel Cav1.3 accelerates the pacemaker rate in the heart, but its functional role for the extended dynamic range of neuronal firing is still unresolved. Here, we show that Cav1.3 calcium channels act as unexpectedly simple, full-range linear amplifiers of firing rates for lateral dopamine substantia nigra (DA SN) neurons in mice. This means that they boost in vitro or in vivo firing frequencies between 2 and 50 hertz by about 30%. Furthermore, we demonstrate that clinically relevant, low nanomolar concentrations of the L-type channel inhibitor isradipine selectively reduce the in vivo firing activity of these nigrostriatal DA SN neurons at therapeutic plasma concentrations. Thus, our study identifies the pacemaker function of neuronal Cav1.3 channels and provides direct evidence that repurposing dihydropyridines such as isradipine is feasible to selectively modulate the in vivo activity of highly vulnerable DA SN subpopulations in Parkinson's disease.
Figures
References
-
- Berger S. M., Bartsch D., The role of L-type voltage-gated calcium channels Cav1.2 and Cav1.3 in normal and pathological brain function. Cell Tissue Res. 357, 463–476 (2014). - PubMed
-
- Striessnig J., Koschak A., Sinnegger-Brauns M. J., Hetzenauer A., Nguyen N. K., Busquet P., Pelster G., Singewald N., Role of voltage-gated L-type Ca2+ channel isoforms for brain function. Biochem. Soc. Trans. 34, 903–909 (2006). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

