Mosquito saliva enhances virus infection through sialokinin-dependent vascular leakage
- PMID: 35675424
- PMCID: PMC9214539
- DOI: 10.1073/pnas.2114309119
Mosquito saliva enhances virus infection through sialokinin-dependent vascular leakage
Abstract
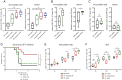
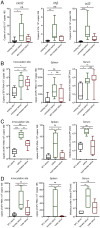
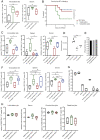
Viruses transmitted by Aedes mosquitoes are an increasingly important global cause of disease. Defining common determinants of host susceptibility to this large group of heterogenous pathogens is key for informing the rational design of panviral medicines. Infection of the vertebrate host with these viruses is enhanced by mosquito saliva, a complex mixture of salivary-gland-derived factors and microbiota. We show that the enhancement of infection by saliva was dependent on vascular function and was independent of most antisaliva immune responses, including salivary microbiota. Instead, the Aedes gene product sialokinin mediated the enhancement of virus infection through a rapid reduction in endothelial barrier integrity. Sialokinin is unique within the insect world as having a vertebrate-like tachykinin sequence and is absent from Anopheles mosquitoes, which are incompetent for most arthropod-borne viruses, whose saliva was not proviral and did not induce similar vascular permeability. Therapeutic strategies targeting sialokinin have the potential to limit disease severity following infection with Aedes-mosquito-borne viruses.
Keywords: arbovirus; endothelium; inflammation; mosquitoes.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

