Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California
- PMID: 35675841
- PMCID: PMC10208005
- DOI: 10.1038/s41591-022-01887-z
Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California
Abstract
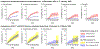
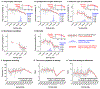
Epidemiologic surveillance has revealed decoupling of Coronavirus Disease 2019 (COVID-19) hospitalizations and deaths from case counts after emergence of the Omicron (B.1.1.529) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant globally. However, assessment of the relative severity of Omicron variant infections presents challenges because of differential acquired immune protection against Omicron and prior variants and because longer-term changes have occurred in testing and healthcare practices. Here we show that Omicron variant infections were associated with substantially reduced risk of progression to severe clinical outcomes relative to time-matched Delta (B.1.617.2) variant infections within a large, integrated healthcare system in Southern California. Adjusted hazard ratios (aHRs) for any hospital admission, symptomatic hospital admission, intensive care unit admission, mechanical ventilation and death comparing individuals with Omicron versus Delta variant infection were 0.59 (95% confidence interval: 0.51-0.69), 0.59 (0.51-0.68), 0.50 (0.29-0.87), 0.36 (0.18-0.72) and 0.21 (0.10-0.44), respectively. This reduced severity could not be explained by differential history of prior infection among individuals with Omicron or Delta variant infection and was starkest among individuals not previously vaccinated against COVID-19 (aHR = 0.40 (0.33-0.49) for any hospital admission and 0.14 (0.07-0.28) for death). Infections with the Omicron BA.2 subvariant were not associated with differential risk of severe outcomes in comparison to BA.1/BA.1.1 subvariant infections. Lower risk of severe clinical outcomes among individuals with Omicron variant infection should inform public health response amid establishment of the Omicron variant as the dominant SARS-CoV-2 lineage globally.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
JAL has received research grants and consulting honoraria unrelated to this study from Pfizer. SYT has received research grants unrelated to this study from Pfizer. ML has received research grants unrelated to this study from Pfizer, and has provided unpaid scientific advisory services to Janssen, Astra-Zeneca, One Day Sooner, and Covaxx (United Biomedical).
Figures
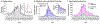


References
-
- World Health Organization. Update on Omicron variant. (2021). Accessed 10 January, 2022. https://www.who.int/news/item/28-11-2021-update-on-omicron
-
- Pearson CAB et al. Bounding the levels of transmissibility and immune evasion of the Omicron variant in South Africa. medRxiv (2021). doi: 10.1101/2021.12.19.21268038. - DOI
-
- Public Health England. SARS-CoV-2 variants of concern and variants under investigation in England—Technical briefing: Update on hospitalisation and vaccine effectiveness for Omicron VOC-21NOV-01 (B.1.1.529). (2021). Accessed 21 February, 2022, https://assets.publishing.service.gov.uk/government/uploads/system/uploa....
Additional references
-
- California Department of Public Health. Variants in California — COVID-19 Response. Accessed 8 May 2022, https://covid19.ca.gov/variants/.
-
- Rubin DB Multiple imputation after 18+ years. J Am Stat Assoc 91, 473–489 (1996).

