Standardised protocol for a prospective cross-sectional multicentre clinical utility evaluation of two dual point-of-care tests in non-clinical settings for the screening of HIV and syphilis in men who have sex with men
- PMID: 35676020
- PMCID: PMC9185395
- DOI: 10.1136/bmjopen-2021-055275
Standardised protocol for a prospective cross-sectional multicentre clinical utility evaluation of two dual point-of-care tests in non-clinical settings for the screening of HIV and syphilis in men who have sex with men
Abstract
Introduction: Point-of-care dual tests (POCTs) for simultaneously detecting antibodies to HIV and syphilis (dual HIV-syphilis POCTs) have been developed recently and show encouraging performance compared with the reference tests in laboratory-based studies. As community-based voluntary, counselling and testing (CBVCT) services are effective providers of HIV and syphilis testing and counselling with high acceptability among men who have sex with men (MSM), the evaluation of the utility of these dual tests in CBVCT services is a high priority. This prospective cross-sectional study will conduct a clinical utility evaluation of two dual POCTs in non-clinical settings for the screening of HIV and syphilis in MSM. This master protocol outlines the overall research approach that will be used in four countries.
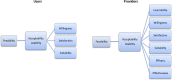
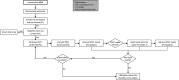
Methods and analysis: MSM presenting at CBVCT services participating in the study for HIV/STI screening will be enrolled. The (WHO preapproved) dual POCTs to be evaluated will be SD Bioline HIV/Syphilis Duo (Abbot) and Dual Path Platform HIV-Syphilis Assay (Chembio). Trained staff will collect a capillary blood sample using finger prick blood to perform both POCTs according the manufacturers' instructions. An analysis of the feasibility of introducing the dual POCT for the screening of HIV and syphilis in MSM at CBVCT services will be performed, by assessing its acceptability and usability at CBVCT service among MSM users and providers.
Ethics and dissemination: This core protocol was independently peer reviewed and approved by the Research Project Review Panel (RP2) of the WHO Department of Sexual and Reproductive Health and Research and by the WHO Ethics Review Committee (ERC). The protocol has been adapted to individual countries and approved by RP2, ERC and institutional review boards at each site. Results will be disseminated through peer-reviewed journals and relevant conferences.
Keywords: HIV & AIDS; Infection control; Public health.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: The POCT manufacturers disclose and furnish the WHO with the information and sufficient quantities of the product(s) free of charge in order to enable this evaluation as part of the WHO/RHR STI POC initiative. The WHO is entitled to evaluate and publish the trial results, and to exclusively control this evaluation and the content of the aforesaid publication. WHO shall submit any proposed publication to the manufacturers for review, comments received will be considered in good faith, but the decision to publish rests with the WHO.
Figures



References
-
- WHO . Latest HIV estimates and updates on HIV policies uptake, November 2020 [Internet], 2020. Available: https://www.who.int/docs/default-source/hiv-hq/latest-hiv-estimates-and-... [Accessed 22 Feb 2021].
-
- UNAIDS data 2020.
-
- European Centre for Disease Prevention and Control, . Who regional office for Europe. HIV/AIDS surveillance in Europe 2020 – 2019 data, 2020. Available: http://apps.who.int/bookorders [Accessed 17 Dec 2020].
-
- ECDC . Syphilis Annual Epidemiological Report for 2018 [Internet], 2019. Available: https://www.ecdc.europa.eu/sites/default/files/documents/syphilis-aer-20... [Accessed 22 Feb 2021].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
