Paramagnetic encoding of molecules
- PMID: 35676253
- PMCID: PMC9177614
- DOI: 10.1038/s41467-022-30811-9
Paramagnetic encoding of molecules
Abstract
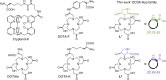
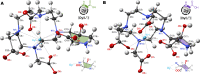
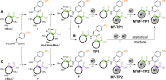
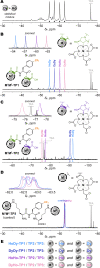
Contactless digital tags are increasingly penetrating into many areas of human activities. Digitalization of our environment requires an ever growing number of objects to be identified and tracked with machine-readable labels. Molecules offer immense potential to serve for this purpose, but our ability to write, read, and communicate molecular code with current technology remains limited. Here we show that magnetic patterns can be synthetically encoded into stable molecular scaffolds with paramagnetic lanthanide ions to write digital code into molecules and their mixtures. Owing to the directional character of magnetic susceptibility tensors, each sequence of lanthanides built into one molecule produces a unique magnetic outcome. Multiplexing of the encoded molecules provides a high number of codes that grows double-exponentially with the number of available paramagnetic ions. The codes are readable by nuclear magnetic resonance in the radiofrequency (RF) spectrum, analogously to the macroscopic technology of RF identification. A prototype molecular system capable of 16-bit (65,535 codes) encoding is presented. Future optimized systems can conceivably provide 64-bit (~10^19 codes) or higher encoding to cover the labelling needs in drug discovery, anti-counterfeiting and other areas.
© 2022. The Author(s).
Conflict of interest statement
M.P. and J.K. are co-inventors on a pending patent application no. PCT/CZ2020/050032 filed in the name of applicant Ustav Organicke Chemie a Biochemie AV CR V.V.I. The application covers some of the compounds discussed in this work. The remaining authors declare no competing interests.
Figures





Similar articles
-
Generation of pseudocontact shifts in proteins with lanthanides using small "clickable" nitrilotriacetic acid and iminodiacetic acid tags.Chemistry. 2015 Mar 23;21(13):5084-92. doi: 10.1002/chem.201406274. Epub 2015 Feb 12. Chemistry. 2015. PMID: 25676727
-
[Ln(DPA)(3)](3-) is a convenient paramagnetic shift reagent for protein NMR studies.J Am Chem Soc. 2009 Aug 5;131(30):10352-3. doi: 10.1021/ja9034957. J Am Chem Soc. 2009. PMID: 19585996
-
Paramagnetic labelling of proteins and oligonucleotides for NMR.J Biomol NMR. 2010 Jan;46(1):101-12. doi: 10.1007/s10858-009-9331-1. Epub 2009 Jun 16. J Biomol NMR. 2010. PMID: 19529883 Review.
-
Site-Specific Tagging of Proteins with Paramagnetic Ions for Determination of Protein Structures in Solution and in Cells.Acc Chem Res. 2019 Jun 18;52(6):1675-1686. doi: 10.1021/acs.accounts.9b00132. Epub 2019 May 31. Acc Chem Res. 2019. PMID: 31150202
-
Pseudocontact shifts in biomolecular NMR using paramagnetic metal tags.Prog Nucl Magn Reson Spectrosc. 2017 Feb;98-99:20-49. doi: 10.1016/j.pnmrs.2016.11.001. Epub 2016 Dec 1. Prog Nucl Magn Reson Spectrosc. 2017. PMID: 28283085 Review. No abstract available.
Cited by
-
Nondestructive Sequencing of Enantiopure Oligoesters by Nuclear Magnetic Resonance Spectroscopy.JACS Au. 2022 Aug 15;2(9):2108-2118. doi: 10.1021/jacsau.2c00388. eCollection 2022 Sep 26. JACS Au. 2022. PMID: 36186555 Free PMC article.
-
Ultra-inert lanthanide chelates as mass tags for multiplexed bioanalysis.Nat Commun. 2024 Nov 13;15(1):9836. doi: 10.1038/s41467-024-53867-1. Nat Commun. 2024. PMID: 39537622 Free PMC article.
-
Super-multiplexed imaging and coding in the range of radio frequency.Nat Commun. 2025 Mar 15;16(1):2567. doi: 10.1038/s41467-025-57785-8. Nat Commun. 2025. PMID: 40089513 Free PMC article.
-
Harnessing Dynamic Supramolecular Interactions for Lanthanide Detection via Computational Pattern Recognition of Magnetic Resonance Fingerprints.J Am Chem Soc. 2025 Jun 4;147(22):18972-18981. doi: 10.1021/jacs.5c03583. Epub 2025 May 19. J Am Chem Soc. 2025. PMID: 40389359 Free PMC article.
-
Probing substrate binding inside a paramagnetic cavity: a NMR spectroscopy toolbox for combined experimental and theoretical investigation.Chem Sci. 2024 Sep 24;15(42):17407-17. doi: 10.1039/d4sc05432f. Online ahead of print. Chem Sci. 2024. PMID: 39364070 Free PMC article.
References
-
- Binan L, Drobetsky EA, Costantino S. Exploiting molecular barcodes in high-throughput cellular assays. SLAS Technol. Transl. Life Sci. Innov. 2019;24:298–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

