Screening and purification of NanB sialidase from Pasteurella multocida with activity in hydrolyzing sialic acid Neu5Acα(2-6)Gal and Neu5Acα(2-3)Gal
- PMID: 35676312
- PMCID: PMC9177577
- DOI: 10.1038/s41598-022-13635-x
Screening and purification of NanB sialidase from Pasteurella multocida with activity in hydrolyzing sialic acid Neu5Acα(2-6)Gal and Neu5Acα(2-3)Gal
Abstract
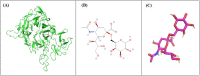
Study on sialidases as antiviral agents has been widely performed, but many types of sialidase have not been tested for their antiviral activity. Pasteurella multocida NanB sialidase is one such sialidase that has never been isolated for further research. In this study, the activity of NanB sialidase was investigated in silico by docking the NanB sialidase of Pasteurella multocida to the Neu5Acα(2-6)Gal and Neu5Acα(2-3)Gal ligands. Additionally, some local isolates of Pasteurella multocida, which had the NanB gene were screened, and the proteins were isolated for further testing regarding their activity in hydrolyzing Neu5Acα(2-6)Gal and Neu5Acα(2-3)Gal. Silico studies showed that the NanB sialidase possesses an exceptional affinity towards forming a protein-ligand complex with Neu5Acα(2-6)Gal and Neu5Acα(2-3)Gal. NanB sialidase of Pasteurella multocida B018 at 0.129 U/mL and 0.258 U/mL doses can hydrolyze Neu5Acα(2-6)Gal and Neu5Acα(2-3)Gal better than other doses. In addition, those doses can inhibit effectively H9N2 viral binding to red blood cells. This study suggested that the NanB sialidase of Pasteurella multocida B018 has a potent antiviral activity because can hydrolyze sialic acid on red blood cells surface and inhibit the H9N2 viral binding to the cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
In vitro antiviral activity of NanB bacterial sialidase against avian influenza H9N2 virus in MDCK cells.Avian Pathol. 2025 Feb;54(1):96-107. doi: 10.1080/03079457.2024.2386315. Epub 2024 Aug 21. Avian Pathol. 2025. PMID: 39069790
-
Cloning and characterization of sialidases with 2-6' and 2-3' sialyl lactose specificity from Pasteurella multocida.J Bacteriol. 2000 Dec;182(24):6874-83. doi: 10.1128/JB.182.24.6874-6883.2000. J Bacteriol. 2000. PMID: 11092845 Free PMC article.
-
A Pasteurella multocida sialyltransferase displaying dual trans-sialidase activities for production of 3'-sialyl and 6'-sialyl glycans.J Biotechnol. 2014 Jan 20;170:60-7. doi: 10.1016/j.jbiotec.2013.11.013. Epub 2013 Nov 27. J Biotechnol. 2014. PMID: 24291191
-
Imaging of Sialidase Activity and Its Clinical Application.Biol Pharm Bull. 2017;40(12):2015-2023. doi: 10.1248/bpb.b17-00592. Biol Pharm Bull. 2017. PMID: 29199226 Review.
-
High efficiency method of detection and isolation of neuraminidase inhibitor resistant influenza viruses by fluorescence sialidase imaging.Jpn J Antibiot. 2016 Dec;69(6):357-366. Jpn J Antibiot. 2016. PMID: 30226930 Review. English, Japanese.
Cited by
-
The Oil Formulation Derived from Moringa Oleifera Seeds Ameliorates Behavioral Abnormalities in Water-immersion Restraint Stress Mouse Model.J Exp Pharmacol. 2022 Dec 22;14:395-407. doi: 10.2147/JEP.S386745. eCollection 2022. J Exp Pharmacol. 2022. PMID: 36583146 Free PMC article.
-
Acute and Subacute Toxicity of Sialidase From Clostridium perfringens Type A in Mice (Mus musculus): Organ-Specific Damage and Immune Response.Vet Med Int. 2025 Jul 1;2025:5582663. doi: 10.1155/vmi/5582663. eCollection 2025. Vet Med Int. 2025. PMID: 40630821 Free PMC article.
-
Structural and functional characterization of cold-active sialidase isolated from Antarctic fungus Penicillium griseofulvum P29.Biochem Biophys Rep. 2023 Dec 20;37:101610. doi: 10.1016/j.bbrep.2023.101610. eCollection 2024 Mar. Biochem Biophys Rep. 2023. PMID: 38155944 Free PMC article.
-
Glucose Catabolite Repression Participates in the Regulation of Sialidase Biosynthesis by Antarctic Strain Penicillium griseofulvum P29.J Fungi (Basel). 2024 Mar 23;10(4):241. doi: 10.3390/jof10040241. J Fungi (Basel). 2024. PMID: 38667912 Free PMC article.
-
Analyzing Molecular Traits of H9N2 Avian Influenza Virus Isolated from a Same Poultry Farm in West Java Province, Indonesia, in 2017 and 2023.F1000Res. 2025 Mar 5;13:571. doi: 10.12688/f1000research.150975.3. eCollection 2024. F1000Res. 2025. PMID: 39610402 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

