Seasonal and sex-dependent gene expression in emu (Dromaius novaehollandiae) fat tissues
- PMID: 35676317
- PMCID: PMC9177602
- DOI: 10.1038/s41598-022-13681-5
Seasonal and sex-dependent gene expression in emu (Dromaius novaehollandiae) fat tissues
Abstract
Emu (Dromaius novaehollandiae) farming has been gaining wide interest for fat production. Oil rendered from this large flightless bird's fat is valued for its anti-inflammatory and antioxidant properties for uses in therapeutics and cosmetics. We analyzed the seasonal and sex-dependent differentially expressed (DE) genes involved in fat metabolism in emus. Samples were taken from back and abdominal fat tissues of a single set of four male and four female emus in April, June, and November for RNA-sequencing. We found 100 DE genes (47 seasonally in males; 34 seasonally in females; 19 between sexes). Seasonally DE genes with significant difference between the sexes in gene ontology terms suggested integrin beta chain-2 (ITGB2) influences fat changes, in concordance with earlier studies. Six seasonally DE genes functioned in more than two enriched pathways (two female: angiopoietin-like 4 (ANGPTL4) and lipoprotein lipase (LPL); four male: lumican (LUM), osteoglycin (OGN), aldolase B (ALDOB), and solute carrier family 37 member 2 (SLC37A2)). Two sexually DE genes, follicle stimulating hormone receptor (FSHR) and perilipin 2 (PLIN2), had functional investigations supporting their influence on fat gain and loss. The results suggested these nine genes influence fat metabolism and deposition in emus.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

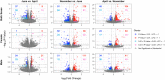

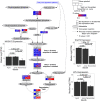

Similar articles
-
Seasonal and sexual variation in mRNA expression of selected adipokine genes affecting fat deposition and metabolism of the emu (Dromaius novaehollandiae).Sci Rep. 2022 Apr 15;12(1):6325. doi: 10.1038/s41598-022-10232-w. Sci Rep. 2022. PMID: 35428830 Free PMC article.
-
Microbial diversity and metabolic function in duodenum, jejunum and ileum of emu (Dromaius novaehollandiae).Sci Rep. 2023 Mar 18;13(1):4488. doi: 10.1038/s41598-023-31684-8. Sci Rep. 2023. PMID: 36934111 Free PMC article.
-
Expressed sequence tag analysis of the emu (Dromaius novaehollandiae) pituitary by 454 GS Junior pyrosequencing.Poult Sci. 2013 Jan;92(1):90-6. doi: 10.3382/ps.2012-02594. Poult Sci. 2013. PMID: 23243234
-
The influence of age and gender on emu (Dromaius novaehollandiae) fat.Sci Rep. 2020 Jul 6;10(1):11082. doi: 10.1038/s41598-020-68103-1. Sci Rep. 2020. PMID: 32632331 Free PMC article.
-
Review on emu products for use as complementary and alternative medicine.Nutrition. 2015 Jan;31(1):21-7. doi: 10.1016/j.nut.2014.04.004. Epub 2014 Apr 19. Nutrition. 2015. PMID: 25441585 Review.
Cited by
-
A PLIN1 polymorphism is associated with fat production in male emus.Poult Sci. 2024 Dec;103(12):104513. doi: 10.1016/j.psj.2024.104513. Epub 2024 Nov 5. Poult Sci. 2024. PMID: 39541877 Free PMC article.
-
Chromosome-level reference genome assembly of the gyrfalcon (Falco rusticolus) and population genomics offer insights into the falcon population in Mongolia.Sci Rep. 2025 Feb 4;15(1):4154. doi: 10.1038/s41598-025-88216-9. Sci Rep. 2025. PMID: 39900672 Free PMC article.
-
Genetic structure and origin of emu populations in Japanese farms inferred from large-scale SNP genotyping based on double-digest RAD-seq.Sci Rep. 2024 Mar 24;14(1):6982. doi: 10.1038/s41598-024-57032-y. Sci Rep. 2024. PMID: 38523157 Free PMC article.
-
Seasonal and sexual variation in mRNA expression of selected adipokine genes affecting fat deposition and metabolism of the emu (Dromaius novaehollandiae).Sci Rep. 2022 Apr 15;12(1):6325. doi: 10.1038/s41598-022-10232-w. Sci Rep. 2022. PMID: 35428830 Free PMC article.
-
Parallel and convergent evolution in genes underlying seasonal migration.Evol Lett. 2024 Nov 30;9(2):189-208. doi: 10.1093/evlett/qrae064. eCollection 2025 Apr. Evol Lett. 2024. PMID: 40191407 Free PMC article.
References
-
- Bennett DC, et al. Comparison of the antioxidant properties of emu oil with other avian oils. Aust. J. Exp. Agric. 2008;48(10):1345–1350. doi: 10.1071/EA08134. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

