RB loss determines selective resistance and novel vulnerabilities in ER-positive breast cancer models
- PMID: 35676324
- PMCID: PMC10680093
- DOI: 10.1038/s41388-022-02362-2
RB loss determines selective resistance and novel vulnerabilities in ER-positive breast cancer models
Abstract
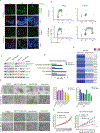
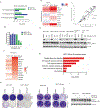
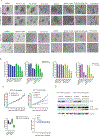
The management of metastatic estrogen receptor (ER) positive HER2 negative breast cancer (ER+) has improved; however, therapeutic resistance and disease progression emerges in majority of cases. Using unbiased approaches, as expected PI3K and MTOR inhibitors emerge as potent inhibitors to delay proliferation of ER+ models harboring PIK3CA mutations. However, the cytostatic efficacy of these drugs is hindered due to marginal impact on the expression of cyclin D1. Different combination approaches involving the inhibition of ER pathway or cell cycle result in durable growth arrest via RB activation and subsequent inhibition of CDK2 activity. However, cell cycle alterations due to RB loss or ectopic CDK4/cyclin D1 activation yields resistance to these cytostatic combination treatments. To define means to counter resistance to targeted therapies imparted with RB loss; complementary drug screens were performed with RB-deleted isogenic cell lines. In this setting, RB loss renders ER+ breast cancer models more vulnerable to drugs that target DNA replication and mitosis. Pairwise combinations using these classes of drugs defines greater selectivity for RB deficiency. The combination of AURK and WEE1 inhibitors, yields synergistic cell death selectively in RB-deleted ER+ breast cancer cells via apoptosis and yields profound disease control in vivo. Through unbiased efforts the XIAP/CIAP inhibitor birinapant was identified as a novel RB-selective agent. Birinapant further enhances the cytotoxic effect of chemotherapies and targeted therapies used in the treatment of ER+ breast cancer models selectively in the RB-deficient setting. Using organoid culture and xenograft models, we demonstrate the highly selective use of birinapant based combinations for the treatment of RB-deficient tumors. Together, these data illustrate the critical role of RB-pathway in response to many agents used to treat ER+ breast cancer, whilst informing new therapeutic approaches that could be deployed against resistant disease.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
ESK and AKW have received research funding from Eli Lilly, Novartis and Pfizer over the last 5 years. There is no current research support from these entities and the study was written in the absence of input from any pharmaceutical company.
Figures




Similar articles
-
Resistance to cyclin-dependent kinase (CDK) 4/6 inhibitors confers cross-resistance to other CDK inhibitors but not to chemotherapeutic agents in breast cancer cells.Breast Cancer. 2021 Jan;28(1):206-215. doi: 10.1007/s12282-020-01150-8. Epub 2020 Aug 28. Breast Cancer. 2021. PMID: 32860163 Free PMC article.
-
Elacestrant (RAD1901) exhibits anti-tumor activity in multiple ER+ breast cancer models resistant to CDK4/6 inhibitors.Breast Cancer Res. 2019 Dec 18;21(1):146. doi: 10.1186/s13058-019-1230-0. Breast Cancer Res. 2019. PMID: 31852484 Free PMC article.
-
Combined Inhibition of mTOR and CDK4/6 Is Required for Optimal Blockade of E2F Function and Long-term Growth Inhibition in Estrogen Receptor-positive Breast Cancer.Mol Cancer Ther. 2018 May;17(5):908-920. doi: 10.1158/1535-7163.MCT-17-0537. Epub 2018 Feb 26. Mol Cancer Ther. 2018. PMID: 29483206 Free PMC article.
-
Mechanism of resistance to endocrine therapy in breast cancer: the important role of PI3K/Akt/mTOR in estrogen receptor-positive, HER2-negative breast cancer.Breast Cancer. 2018 Jul;25(4):392-401. doi: 10.1007/s12282-017-0812-x. Epub 2017 Oct 31. Breast Cancer. 2018. PMID: 29086897 Review.
-
Mechanisms of resistance to estrogen receptor modulators in ER+/HER2- advanced breast cancer.Cell Mol Life Sci. 2020 Feb;77(4):559-572. doi: 10.1007/s00018-019-03281-4. Epub 2019 Aug 30. Cell Mol Life Sci. 2020. PMID: 31471681 Free PMC article. Review.
Cited by
-
Research progress and application status of organoid in breast cancer subtypes.Biomol Biomed. 2025 Apr 3;25(5):976-985. doi: 10.17305/bb.2024.11450. Biomol Biomed. 2025. PMID: 39720912 Free PMC article. Review.
-
Rbf/E2F1 control growth and endoreplication via steroid-independent Ecdysone Receptor signalling in Drosophila prostate-like secondary cells.PLoS Genet. 2023 Jun 26;19(6):e1010815. doi: 10.1371/journal.pgen.1010815. eCollection 2023 Jun. PLoS Genet. 2023. PMID: 37363926 Free PMC article.
-
The BET inhibitor/degrader ARV-825 prolongs the growth arrest response to Fulvestrant + Palbociclib and suppresses proliferative recovery in ER-positive breast cancer.Front Oncol. 2023 Jan 18;12:966441. doi: 10.3389/fonc.2022.966441. eCollection 2022. Front Oncol. 2023. PMID: 36741704 Free PMC article.
-
CDK4/6 inhibitor resistance in estrogen receptor positive breast cancer, a 2023 perspective.Front Cell Dev Biol. 2023 Mar 22;11:1148792. doi: 10.3389/fcell.2023.1148792. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37035239 Free PMC article. Review.
-
BH3 mimetics targeting BCL-XL have efficacy in solid tumors with RB1 loss and replication stress.Nat Commun. 2025 May 28;16(1):4931. doi: 10.1038/s41467-025-60238-x. Nat Commun. 2025. PMID: 40436896 Free PMC article.
References
-
- Ahmad A. Breast cancer statistics: recent trends. Adv Exp Med Biol. 2019;1152:1–7. - PubMed
-
- Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 2003;9:1980–9. - PubMed
-
- Howell A. The endocrine prevention of breast cancer. Best Pract Res Clin Endocrinol Metab. 2008;22:615–23. - PubMed
-
- Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

