Pregnancy enables antibody protection against intracellular infection
- PMID: 35676476
- PMCID: PMC9233044
- DOI: 10.1038/s41586-022-04816-9
Pregnancy enables antibody protection against intracellular infection
Abstract
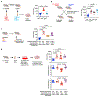
Adaptive immune components are thought to exert non-overlapping roles in antimicrobial host defence, with antibodies targeting pathogens in the extracellular environment and T cells eliminating infection inside cells1,2. Reliance on antibodies for vertically transferred immunity from mothers to babies may explain neonatal susceptibility to intracellular infections3,4. Here we show that pregnancy-induced post-translational antibody modification enables protection against the prototypical intracellular pathogen Listeria monocytogenes. Infection susceptibility was reversed in neonatal mice born to preconceptually primed mothers possessing L. monocytogenes-specific IgG or after passive transfer of antibodies from primed pregnant, but not virgin, mice. Although maternal B cells were essential for producing IgGs that mediate vertically transferred protection, they were dispensable for antibody acquisition of protective function, which instead required sialic acid acetyl esterase5 to deacetylate terminal sialic acid residues on IgG variable-region N-linked glycans. Deacetylated L. monocytogenes-specific IgG protected neonates through the sialic acid receptor CD226,7, which suppressed IL-10 production by B cells leading to antibody-mediated protection. Consideration of the maternal-fetal dyad as a joined immunological unit reveals protective roles for antibodies against intracellular infection and fine-tuned adaptations to enhance host defence during pregnancy and early life.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Reporting summary
Further information on research design in the Nature Research Reporting Summary linked to this paper.
Competing Interests
A patent on antibody sialic acid modification has been filed by Cincinnati Children’s Hospital with J.J.E. and S.S.W. as inventors (PCT/US2022/018847). A.B.H. has equity in Chelexa BioSciences, LLC and in Hoth Therapeutics, Inc., and he serves on the Scientific Advisory Board of Hoth Therapeutics. All other authors declare no competing interests.
Figures











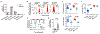

Comment in
-
Pregnancy-specific antibody modification.Nat Rev Immunol. 2022 Aug;22(8):462-463. doi: 10.1038/s41577-022-00752-x. Nat Rev Immunol. 2022. PMID: 35697800 No abstract available.
-
Only pregnant moms can do it….Sci Immunol. 2022 Jul;7(73):eadd6618. doi: 10.1126/sciimmunol.add6618. Epub 2022 Jul 1. Sci Immunol. 2022. PMID: 35776805
References
MeSH terms
Substances
Grants and funding
- F32 AI145184/AI/NIAID NIH HHS/United States
- R01 AI124657/AI/NIAID NIH HHS/United States
- R01 AI107250/AI/NIAID NIH HHS/United States
- R01 AI145840/AI/NIAID NIH HHS/United States
- R01 GM094363/GM/NIGMS NIH HHS/United States
- DP1 AI131080/AI/NIAID NIH HHS/United States
- R01 AI120202/AI/NIAID NIH HHS/United States
- R01 AI162964/AI/NIAID NIH HHS/United States
- P01 HL151333/HL/NHLBI NIH HHS/United States
- T32 DK007727/DK/NIDDK NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- U01 AI144673/AI/NIAID NIH HHS/United States
- R24 GM137782/GM/NIGMS NIH HHS/United States
- K12 HD028827/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

