A preoptic neuronal population controls fever and appetite during sickness
- PMID: 35676482
- PMCID: PMC9327738
- DOI: 10.1038/s41586-022-04793-z
A preoptic neuronal population controls fever and appetite during sickness
Abstract
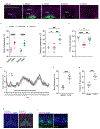
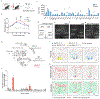
During infection, animals exhibit adaptive changes in physiology and behaviour aimed at increasing survival. Although many causes of infection exist, they trigger similar stereotyped symptoms such as fever, warmth-seeking, loss of appetite and fatigue1,2. Yet exactly how the nervous system alters body temperature and triggers sickness behaviours to coordinate responses to infection remains unknown. Here we identify a previously uncharacterized population of neurons in the ventral medial preoptic area (VMPO) of the hypothalamus that are activated after sickness induced by lipopolysaccharide (LPS) or polyinosinic:polycytidylic acid. These neurons are crucial for generating a fever response and other sickness symptoms such as warmth-seeking and loss of appetite. Single-nucleus RNA-sequencing and multiplexed error-robust fluorescence in situ hybridization uncovered the identity and distribution of LPS-activated VMPO (VMPOLPS) neurons and non-neuronal cells. Gene expression and electrophysiological measurements implicate a paracrine mechanism in which the release of immune signals by non-neuronal cells during infection activates nearby VMPOLPS neurons. Finally, we show that VMPOLPS neurons exert a broad influence on the activity of brain areas associated with behavioural and homeostatic functions and are synaptically and functionally connected to circuit nodes controlling body temperature and appetite. Together, these results uncover VMPOLPS neurons as a control hub that integrates immune signals to orchestrate multiple sickness symptoms in response to infection.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing financial interests
X.Z. is an inventor on patents applied for by Harvard University related to MERFISH.
Figures









Comment in
-
Coordinating sickness behaviour.Nat Rev Neurosci. 2022 Aug;23(8):456-457. doi: 10.1038/s41583-022-00616-2. Nat Rev Neurosci. 2022. PMID: 35732916 No abstract available.
-
Neuroscience: Detection of systemic inflammation by the brain.Curr Biol. 2022 Jul 11;32(13):R751-R753. doi: 10.1016/j.cub.2022.05.028. Curr Biol. 2022. PMID: 35820388
-
Neuronal culprits of sickness behaviours.Nature. 2022 Sep;609(7928):679-680. doi: 10.1038/d41586-022-02321-7. Nature. 2022. PMID: 36071222 No abstract available.
References
-
- Konsman JP, Parnet P & Dantzer R Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci 25, 154–159, (2002). - PubMed
-
- Quan N & Banks WA Brain-immune communication pathways. Brain Behav Immun 21, 727–735, (2007). - PubMed
-
- Nakamori T et al. Organum vasculosum laminae terminalis (OVLT) is a brain site to produce interleukin-1 beta during fever. Brain Res 618, 155–159, (1993). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

