Potentiating adoptive cell therapy using synthetic IL-9 receptors
- PMID: 35676488
- PMCID: PMC9283313
- DOI: 10.1038/s41586-022-04801-2
Potentiating adoptive cell therapy using synthetic IL-9 receptors
Erratum in
-
Publisher Correction: Potentiating adoptive cell therapy using synthetic IL-9 receptors.Nature. 2022 Dec;612(7938):E10. doi: 10.1038/s41586-022-05548-6. Nature. 2022. PMID: 36380037 Free PMC article. No abstract available.
Abstract
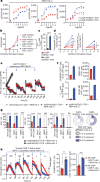
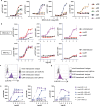
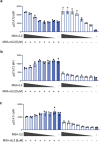
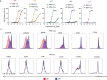
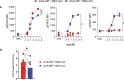
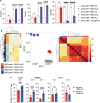
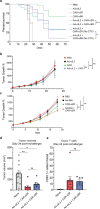
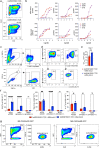
Synthetic receptor signalling has the potential to endow adoptively transferred T cells with new functions that overcome major barriers in the treatment of solid tumours, including the need for conditioning chemotherapy1,2. Here we designed chimeric receptors that have an orthogonal IL-2 receptor extracellular domain (ECD) fused with the intracellular domain (ICD) of receptors for common γ-chain (γc) cytokines IL-4, IL-7, IL-9 and IL-21 such that the orthogonal IL-2 cytokine elicits the corresponding γc cytokine signal. Of these, T cells that signal through the chimeric orthogonal IL-2Rβ-ECD-IL-9R-ICD (o9R) are distinguished by the concomitant activation of STAT1, STAT3 and STAT5 and assume characteristics of stem cell memory and effector T cells. Compared to o2R T cells, o9R T cells have superior anti-tumour efficacy in two recalcitrant syngeneic mouse solid tumour models of melanoma and pancreatic cancer and are effective even in the absence of conditioning lymphodepletion. Therefore, by repurposing IL-9R signalling using a chimeric orthogonal cytokine receptor, T cells gain new functions, and this results in improved anti-tumour activity for hard-to-treat solid tumours.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
A.K. serves on the advisory board of T-Cure Therapeutics and Certis Oncology, holds stock in Certis Oncology, and receives research funding from Highlight Therapeutics. K.C.G., L.L.S., and L.K.P. are inventors on patent application PCT/US2020/050232 on the ortho-9 technology and are shareholders in Synthekine Therapeutics. K.C.G. is the founder of Synthekine Therapeutics. A.R. has received honoraria for consulting with Amgen, Bristol-Myers Squibb, Chugai, Genentech, Merck, Novartis, Roche and Sanofi, is or has been a member of the scientific advisory board and holds stock in Advaxis, Arcus Biosciences, Highlight Therapeutics, Compugen, CytomX, Five Prime, FLX Bio, ImaginAb, IsoPlexis, Gilead Kite, Lutris Pharma, Merus, PACT Pharma, Rgenix and Tango Therapeutics. C.H.J. has received honoraria for consulting with, is a member of the board of directors for AC Immune, is a member of the scientific advisory board and holds stock in Bluesphere Bio, Cabaletta Bio, Carisma Therapeutics, Cellares, Celldex, DeCART Therapeutics, Kiadis Pharma, Tmunity Therapeutics, WIRB-Copernicus Group and Ziopharm Oncology, and receives royalties for intellectual property licensed to Novartis and Tmunity.
Figures






Comment in
-
Fine-tuning T cell function through engineered orthogonal chimeric cytokine receptors.Cell Res. 2022 Nov;32(11):967-968. doi: 10.1038/s41422-022-00715-w. Cell Res. 2022. PMID: 36002586 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

