Functional and phylogenetic analyses of camel rumen microbiota associated with different lignocellulosic substrates
- PMID: 35676509
- PMCID: PMC9177762
- DOI: 10.1038/s41522-022-00309-9
Functional and phylogenetic analyses of camel rumen microbiota associated with different lignocellulosic substrates
Abstract
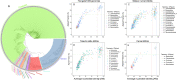
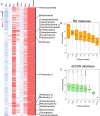
Rumen microbiota facilitates nutrition through digestion of recalcitrant lignocellulosic substrates into energy-accessible nutrients and essential metabolites. Despite the high similarity in rumen microbiome structure, there might be distinct functional capabilities that enable different ruminant species to thrive on various lignocellulosic substrates as feed. Here, we applied genome-centric metagenomics to explore phylogenetic diversity, lignocellulose-degrading potential and fermentation metabolism of biofilm-forming microbiota colonizing 11 different plant substrates in the camel rumen. Diversity analysis revealed significant variations in the community of rumen microbiota colonizing different substrates in accordance with their varied physicochemical properties. Metagenome reconstruction recovered genome sequences of 590 bacterial isolates and one archaeal lineage belonging to 20 microbial phyla. A comparison to publicly available reference genomes and rumen metagenome-assembled genomes revealed that most isolates belonged to new species with no well-characterized representatives. We found that certain low abundant taxa, including members of Verrucomicrobiota, Planctomycetota and Fibrobacterota, possessed a disproportionately large number of carbohydrate active enzymes per Mb of genome, implying their high metabolic potential to contribute to the rumen function. In conclusion, we provided a detailed picture of the diversity and functional significance of rumen microbiota colonizing feeds of varying lignocellulose composition in the camel rumen. A detailed analysis of 591 metagenome-assembled genomes revealed a network of interconnected microbiota and highlighted the key roles of certain taxonomic clades in rumen function, including those with minimal genomes (e.g., Patescibacteria). The existence of a diverse array of gene clusters encoding for secondary metabolites unveiled the specific functions of these biomolecules in shaping community structure of rumen microbiota.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

