Phoenixin-14 alters transcriptome and steroid profiles in female green-spotted puffer (Dichotomyctere nigroviridis)
- PMID: 35676522
- PMCID: PMC9177834
- DOI: 10.1038/s41598-022-13695-z
Phoenixin-14 alters transcriptome and steroid profiles in female green-spotted puffer (Dichotomyctere nigroviridis)
Abstract
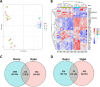
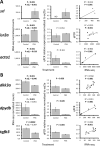
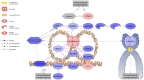
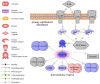
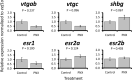
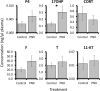
Phoenixin (PNX) is a highly conserved, novel hormone with diverse functions, including hypothalamic control of reproduction, appetite modulation, and regulation of energy metabolism and inflammation. While some functions appear conserved across vertebrates, additional research is required to fully characterize these complex pleiotropic effects. For instance, very little is known about transcriptome level changes associated with PNX exposure, including responses in the hypothalamic-pituitary-gonadal (HPG) axis, which is critical in vertebrate reproduction. In addition, the PNX system may be especially complex in fish, where an additional receptor is likely present in some species. The purpose of this study was to assess hypothalamic and ovarian transcriptomes after PNX-14 administration in female vitellogenic green-spotted puffer (Dichotomyctere nigroviridis). Steroid-related changes were also assessed in the liver and blood plasma. Hypothalamic responses included pro-inflammatory signals such as interleukin 1β, possibly related to gut-brain axis functions, as well as suppression of cell proliferation. Ovarian responses were more widely downregulated across all identified pathways, which may reflect progression to a less transcriptionally active state in oocytes. Both organs shared regulation in transforming growth factor-β and extracellular matrix remodeling (periostin) pathways. Reproductive processes were in general downregulated, but both inhibiting (bone morphogenetic protein 15 and follistatin) and promoting (17-hydroxyprogesterone) factors for oocyte maturation were identified. Select genes involved in reproduction (vitellogenins, estrogen receptors) in the liver were unresponsive to PNX-14 and higher doses may be needed to induce reproductive effects in D. nigroviridis. These results reinforce the complexity of PNX actions in diverse tissues and highlight important roles for this hormone in regulating the immune response, energy metabolism, and cell growth.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Phoenixin participated in regulation of food intake and growth in spotted scat, Scatophagus argus.Comp Biochem Physiol B Biochem Mol Biol. 2018 Dec;226:36-44. doi: 10.1016/j.cbpb.2018.07.007. Epub 2018 Aug 13. Comp Biochem Physiol B Biochem Mol Biol. 2018. PMID: 30114526
-
Phoenixin: Expression at different ovarian development stages and effects on genes ralated to reproduction in spotted scat, Scatophagus argus.Comp Biochem Physiol B Biochem Mol Biol. 2019 Feb;228:17-25. doi: 10.1016/j.cbpb.2018.10.005. Epub 2018 Nov 10. Comp Biochem Physiol B Biochem Mol Biol. 2019. PMID: 30423433
-
Phoenixin-20 Stimulates mRNAs Encoding Hypothalamo-Pituitary-Gonadal Hormones, is Pro-Vitellogenic, and Promotes Oocyte Maturation in Zebrafish.Sci Rep. 2020 Apr 14;10(1):6264. doi: 10.1038/s41598-020-63226-x. Sci Rep. 2020. PMID: 32286445 Free PMC article.
-
Phoenixin: A Newly Discovered Peptide with Multi-Functions.Protein Pept Lett. 2017;24(6):472-475. doi: 10.2174/0929866524666170207154417. Protein Pept Lett. 2017. PMID: 28176660 Review.
-
The Regulation of Phoenixin: A Fascinating Multidimensional Peptide.J Endocr Soc. 2021 Dec 24;6(2):bvab192. doi: 10.1210/jendso/bvab192. eCollection 2022 Feb 1. J Endocr Soc. 2021. PMID: 35059547 Free PMC article. Review.
Cited by
-
Characterizing the SREB G protein-coupled receptor family in fish: Brain gene expression and genomic differences in upstream transcription factor binding sites.Comp Biochem Physiol A Mol Integr Physiol. 2023 Nov;285:111507. doi: 10.1016/j.cbpa.2023.111507. Epub 2023 Aug 21. Comp Biochem Physiol A Mol Integr Physiol. 2023. PMID: 37611891 Free PMC article.
-
In silico microRNA network data in zebrafish after antineoplastic ifosfamide exposure.Data Brief. 2023 Mar 29;48:109099. doi: 10.1016/j.dib.2023.109099. eCollection 2023 Jun. Data Brief. 2023. PMID: 37089209 Free PMC article.
-
Spatial and quantitative gene expression analysis of SREB receptors in the gonads of green-spotted pufferfish (Dichotomyctere nigroviridis).Gen Comp Endocrinol. 2025 Jan 1;360:114641. doi: 10.1016/j.ygcen.2024.114641. Epub 2024 Nov 12. Gen Comp Endocrinol. 2025. PMID: 39536984
-
Sub-network transcriptome dataset for diseases associated with exposure to bisphenol F and bisphenol S in human SH-SY5Y neuroblastoma cells.Data Brief. 2025 Jan 21;59:111313. doi: 10.1016/j.dib.2025.111313. eCollection 2025 Apr. Data Brief. 2025. PMID: 39968403 Free PMC article.
-
Phoenixin-14 as a novel direct regulator of porcine luteal cell functions†.Biol Reprod. 2024 Jan 13;110(1):154-168. doi: 10.1093/biolre/ioad138. Biol Reprod. 2024. PMID: 37815939 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

