Jaw1/LRMP increases Ca2+ influx upon GPCR stimulation with heterogeneous effect on the activity of each ITPR subtype
- PMID: 35676525
- PMCID: PMC9177832
- DOI: 10.1038/s41598-022-13620-4
Jaw1/LRMP increases Ca2+ influx upon GPCR stimulation with heterogeneous effect on the activity of each ITPR subtype
Abstract
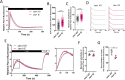
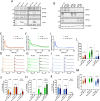
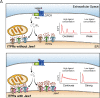
Ca2+ influx upon G protein-coupled receptor (GPCR) stimulation is observed as a cytosolic Ca2+ concentration oscillation crucial to initiating downstream responses including cell proliferation, differentiation, and cell-cell communication. Although Jaw1 is known to interact with inositol 1,4,5-triphosphate receptor (ITPRs), Ca2+ channels on the endoplasmic reticulum, the function of Jaw1 in the Ca2+ dynamics with physiological stimulation remains unclear. In this study, using inducible Jaw1-expressing HEK293 cells, we showed that Jaw1 increases Ca2+ influx by GPCR stimulation via changing the Ca2+ influx oscillation pattern. Furthermore, we showed that Jaw1 increases the Ca2+ release activity of all ITPR subtypes in a subtly different manner. It is well known that the Ca2+ influx oscillation pattern varies from cell type to cell type, therefore these findings provide an insight into the relationship between the heterogeneous Ca2+ dynamics and the specific ITPR and Jaw1 expression patterns.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Jaw1 accelerates the reaction speed of the Ca2+ signals via ITPRs upon GPCR stimulation.Sci Rep. 2025 Mar 24;15(1):10104. doi: 10.1038/s41598-025-94489-x. Sci Rep. 2025. PMID: 40128249 Free PMC article.
-
Cleavage of the Jaw1 C-terminal region enhances its augmentative effect on the Ca2+ release via IP3 receptors.J Cell Sci. 2023 Feb 15;136(4):jcs260439. doi: 10.1242/jcs.260439. Epub 2023 Feb 15. J Cell Sci. 2023. PMID: 36789796
-
Lrmp/Jaw1 is expressed in sweet, bitter, and umami receptor-expressing cells.Chem Senses. 2010 Feb;35(2):171-7. doi: 10.1093/chemse/bjp097. Chem Senses. 2010. PMID: 20071408 Free PMC article.
-
ITPRs/inositol 1,4,5-trisphosphate receptors in autophagy: From enemy to ally.Autophagy. 2015;11(10):1944-8. doi: 10.1080/15548627.2015.1083666. Autophagy. 2015. PMID: 26291777 Free PMC article. Review.
-
From Stores to Sinks: Structural Mechanisms of Cytosolic Calcium Regulation.Adv Exp Med Biol. 2017;981:215-251. doi: 10.1007/978-3-319-55858-5_10. Adv Exp Med Biol. 2017. PMID: 29594864 Review.
Cited by
-
Age and sex drive differential behavioral and neuroimmune phenotypes during postoperative pain.Neurobiol Aging. 2023 Mar;123:129-144. doi: 10.1016/j.neurobiolaging.2022.09.008. Epub 2022 Nov 23. Neurobiol Aging. 2023. PMID: 36577640 Free PMC article.
-
Novel Functional Features of cGMP Substrate Proteins IRAG1 and IRAG2.Int J Mol Sci. 2023 Jun 7;24(12):9837. doi: 10.3390/ijms24129837. Int J Mol Sci. 2023. PMID: 37372987 Free PMC article. Review.
-
Jaw1 accelerates the reaction speed of the Ca2+ signals via ITPRs upon GPCR stimulation.Sci Rep. 2025 Mar 24;15(1):10104. doi: 10.1038/s41598-025-94489-x. Sci Rep. 2025. PMID: 40128249 Free PMC article.
-
Histamine activates an intracellular Ca2+ signal in normal human lung fibroblast WI-38 cells.Front Cell Dev Biol. 2022 Sep 2;10:991659. doi: 10.3389/fcell.2022.991659. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36120576 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

