Comparative efficacy and safety of infliximab and vedolizumab therapy in patients with inflammatory bowel disease: a systematic review and meta-analysis
- PMID: 35676620
- PMCID: PMC9178865
- DOI: 10.1186/s12876-022-02347-1
Comparative efficacy and safety of infliximab and vedolizumab therapy in patients with inflammatory bowel disease: a systematic review and meta-analysis
Abstract
Background and aims: There are limited comparative data for infliximab and vedolizumab in inflammatory bowel disease patients.
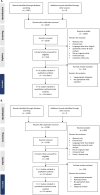
Methods: We conducted a systematic review and meta-analysis to compare the efficacy and safety of infliximab and vedolizumab in adult patients with moderate-to-severe Crohn's disease or ulcerative colitis.
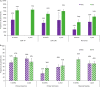
Results: We identified six eligible Crohn's disease and seven eligible ulcerative colitis trials that randomised over 1900 participants per disease cohort to infliximab or vedolizumab. In the Crohn's disease and ulcerative colitis cohorts, infliximab yielded better efficacy than vedolizumab for all analysed outcomes (CDAI-70, CDAI-100 responses, and clinical remission for Crohn's disease and clinical response and clinical remission for ulcerative colitis) during the induction phase, with non-overlapping 95% confidence intervals. In the maintenance phase, similar proportions of infliximab- or vedolizumab-treated patients achieved clinical response, clinical remission, or mucosal healing in both Crohn's disease and ulcerative colitis. For the safety outcomes, rates of adverse events, serious adverse events, and discontinuations due to adverse events were similar in infliximab- and vedolizumab-treated patients in both diseases. The infection rate was higher in infliximab for Crohn's disease and higher in vedolizumab when treating patients with ulcerative colitis. There was no difference between the treatments in the proportions of patients who reported serious infections in both indications.
Conclusions: Indirect comparison of infliximab and vedolizumab trials in adult patients with moderate-to severe Crohn's disease or ulcerative colitis demonstrated that infliximab has better efficacy in the induction phase and comparable efficacy during the maintenance phase and overall safety profile compared to vedolizumab.
Keywords: Antibodies; Biological therapy; Crohn’s disease; IBD; Monoclonal; Tumor Necrosis Factor-alpha; Ulcerative colitis.
© 2022. The Author(s).
Conflict of interest statement
LP received personal fees from Galapagos, AbbVie, Janssen, Genentech, Ferring, Tillots, Pharmacosmos, Celltrion, Takeda, Boerhinger-Ingelheim, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Alma, Sterna, Nestle, Inotrem, Enterome, Allergan, MSD, Roche, Arena, Gilead, Hikma, Amgen, BMS, Vifor, Norgine, Mylan, Lilly, Fresenius Kabi, Oppilan Pharma, Sublimity Therapeutics, Applied Molecular Transport, OSE Immunotherapeutics, Enthera, Theravance. PA has been an advisory board member of Janssen. AA has been a consultant for AbbVie, Allergan, Amgen, Biogen, Bristol Myers Squibb, Celgene, Celltrion, Ferring, Gilead, Janssen, Lilly, MSD, Mylan, Pfizer, Roche, Samsung Bioepis, Sandoz, Takeda; SD has received consultancy fees from AbbVie, Allergan, Amgen, AstraZeneca, Athos Therapeutics, Biogen, Boehringer-Ingelheim, Celgene, Celltrion, Eli Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB Inc., Vifor, and lecture fees from AbbVie, Amgen, Ferring Pharmaceuticals Inc., Gilead, Janssen, Mylan, Pfizer, and Takeda. Jordi Guardiola: Roche, MSD, Abbvie, Kern Pharma, Takeda, Janssen, Pfizer, Ferring, Chiesi, GE Healthcare; JJ has been a speaker, consultant or advisory board member (ABM) for AbbVie, Astro Pharma, Boerhinger-Ingelheim, BMS, Celltrion, Ferring, Giliad, Hikma, Janssen, Meda, MSD, Napp Pharma, Novartis, Orion Pharma, Pfizer, Pharmacosmos, Roche, Takeda, Tillotts, Sandoz, Unimedic Pharma. CL has received research support from Abbvie, Gilead, and Trellus Health; acted as a consultant to Abbvie, Janssen, Takeda, Pfizer, Celltrion, Galapagos, GSK, Gilead, Vifor Pharma, Dr Falk, and Iterative Scopes; he has received speaking fees and travel support from Pfizer, Janssen, Abbvie, Celltion, Galapagos, Takeda, Shire, Ferring and Dr Falk. EL has received research grants from Takeda, Pfizer, Janssen, educational grants from Abbvie, Takeda, Janssen, has received speaker fees from Abbvie, Ferring, MSD, Falk, Takeda, Janssen, Pfizer, Celgene, has been an ABM of Abbvie, Ferring, MSD, Takeda, Celgene, Janssen, Gilead-Galapagos, Arena, Pfizer, Eli Lilly, and a consultant of Abbvie. ML has received financial supports for research and educational activities from Takeda, Janssen, Pfizer, has been an ABM of Janssen, Takeda, Egis. WR has been a speaker for Abbott Laboratories, Abbvie, Aesca, Aptalis, Astellas, Centocor, Celltrion, Danone Austria, Elan, Falk, Ferring, Immundiagnostik, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, PLS Education, Schering-Plough, Shire, Takeda, Therakos, Vifor, Yakult, a consultant for Abbott Laboratories, Abbvie, Aesca, Algernon, Amgen, AM Pharma, AMT, AOP Orphan, Arena Pharmaceuticals, Astellas, Astra Zeneca, Avaxia, Roland Berger GmBH, Bioclinica, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Covance, Danone Austria, DSM, Elan, Eli Lilly, Ernest & Young, Falk, Ferring, Galapagos, Gatehouse Bio Inc., Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Intrinsic Imaging, Janssen, J&J, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, LivaNova, Mallinckrodt, Medahead, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nash Pharmaceuticals, Nestle, Nippon Kayaku, Novartis, Ocera, OMass, Otsuka, Parexel, PDL, Periconsulting, Pharmacosmos, Philip Morris Institute, Pfizer, Procter & Gamble, Prometheus, Protagonist, Provention, Quell Therapeutics, Robarts Clinical Trial, Sandoz, Schering-Plough, Second Genome, Seres Therapeutics, Setpointmedical, Sigmoid, Sublimity, Takeda, Therakos, Theravance, Tigenix, UCB, Vifor, Zealand, Zyngenia, 4SC, an ABM for Abbott Laboratories, Abbvie, Aesca, Amgen, AM Pharma, Astellas, Astra Zeneca, Avaxia, Biogen IDEC, Boehringer-Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Danone Austria, DSM, Elan, Ferring, Galapagos, Genentech, Grünenthal, Inova, Janssen, J&J, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestle, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Sandoz, Schering-Plough, Second Genome, Setpointmedical, Takeda, Therakos, Tigenix, UCB, Zealand, Zyngenia, 4SC, has received research funding from Abbott Laboratories, Abbvie, Aesca, Centocor, Falk, Immundiagnostik, Janssen, MSD, Sandoz, Takeda. XR Celltrion, MSD, Janssen, Takeda, Pfizer, Amgen, Gilead, Tillots
JJ (Jørgen Jahnsen) has served as a speaker, consultant or advisory board member for AbbVie, Astro Pharma, Boerhinger Ingelheim, BMS, Celltrion, Celgene, Ferring, Giliad, Hikma, Janssen, Meda, MSD, Napp Pharma, Novartis, Orion Pharma, Pfizer, Pharmacosmos, Roche, Takeda, Tillotts, Sandoz and Unimedic Pharma.
Figures




References
-
- Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's disease cA2 study group. N Engl J Med. 1997;337(15):1029–1035. doi: 10.1056/NEJM199710093371502. - DOI - PubMed

