Acute effects on glucose tolerance by neprilysin inhibition in patients with type 2 diabetes
- PMID: 35676803
- PMCID: PMC9545540
- DOI: 10.1111/dom.14789
Acute effects on glucose tolerance by neprilysin inhibition in patients with type 2 diabetes
Abstract
Aims: Sacubitril/valsartan is a neprilysin-inhibitor/angiotensin II receptor blocker used for the treatment of heart failure. Recently, a post-hoc analysis of a 3-year randomized controlled trial showed improved glycaemic control with sacubitril/valsartan in patients with heart failure and type 2 diabetes. We previously reported that sacubitril/valsartan combined with a dipeptidyl peptidase-4 inhibitor increases active glucagon-like peptide-1 (GLP-1) in healthy individuals. We now hypothesized that administration of sacubitril/valsartan with or without a dipeptidyl peptidase-4 inhibitor would lower postprandial glucose concentrations (primary outcome) in patients with type 2 diabetes via increased active GLP-1.
Methods: We performed a crossover trial in 12 patients with obesity and type 2 diabetes. A mixed meal was ingested following five respective interventions: (a) a single dose of sacubitril/valsartan; (b) sitagliptin; (c) sacubitril/valsartan + sitagliptin; (d) control (no treatment); and (e) valsartan alone. Glucose, gut and pancreatic hormone responses were measured.
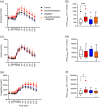
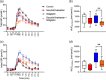
Results: Postprandial plasma glucose increased by 57% (incremental area under the curve 0-240 min) (p = .0003) and increased peak plasma glucose by 1.7 mM (95% CI: 0.6-2.9) (p = .003) after sacubitril/valsartan compared with control, whereas postprandial glucose levels did not change significantly after sacubitril/valsartan + sitagliptin. Glucagon, GLP-1 and C-peptide concentrations increased after sacubitril/valsartan, but insulin and glucose-dependent insulinotropic polypeptide did not change.
Conclusions: The glucose-lowering effects of long-term sacubitril/valsartan treatment reported in patients with heart failure and type 2 diabetes may not depend on changes in entero-pancreatic hormones. Neprilysin inhibition results in hyperglucagonaemia and this may explain the worsen glucose tolerance observed in this study.
Clinicaltrials: gov (NCT03893526).
Keywords: GLP-1; clinical trial; drug mechanism; glucagon; glycaemic control.
© 2022 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
NJWA has received speaker fees from MSD as subsidiary of MERCK and Mercodia, research support from Novo Nordisk A/S and Mercodia. CFD has received consultancy/lecture fees from companies with an interest in developing and marketing incretin‐based therapies for treatment of type 2 diabetes (Boehringer Ingelheim, Lilly, Merck/MSD, Novo Nordisk). Spouse holds stock in Merck/MSD. JJH and LLG are members of advisory boards for Novo Nordisk A/S and has received lecture fees from the same company. Other authors declare that they have no competing interests.
Figures

References
-
- Seferovic JP, Claggett B, Seidelmann SB, et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post‐hoc analysis from the PARADIGM‐HF trial. Lancet Diabetes Endocrinol. 2017;5(5):333‐340. doi: 10.1016/s2213-8587(17)30087-6 - DOI - PMC - PubMed
-
- Erdös EG, Skidgel RA. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989;3(2):145‐151. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

