In vivo and in vitro characterization of GL0034, a novel long-acting glucagon-like peptide-1 receptor agonist
- PMID: 35676825
- PMCID: PMC9796023
- DOI: 10.1111/dom.14794
In vivo and in vitro characterization of GL0034, a novel long-acting glucagon-like peptide-1 receptor agonist
Abstract
Aims: To describe the in vitro characteristics and antidiabetic in vivo efficacy of the novel glucagon-like peptide-1 receptor agonist (GLP-1RA) GL0034.
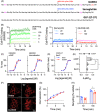
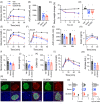
Materials and methods: Glucagon-like peptide-1 receptor (GLP-1R) kinetic binding parameters, cyclic adenosine monophosphate (cAMP) signalling, endocytosis and recycling were measured using HEK293 and INS-1832/3 cells expressing human GLP-1R. Insulin secretion was measured in vitro using INS-1832/3 cells, mouse islets and human islets. Chronic administration studies to evaluate weight loss and glycaemic effects were performed in db/db and diet-induced obese mice.
Results: Compared to the leading GLP-1RA semaglutide, GL0034 showed increased binding affinity and potency-driven bias in favour of cAMP over GLP-1R endocytosis and β-arrestin-2 recruitment. Insulin secretory responses were similar for both ligands. GL0034 (6 nmol/kg) led to at least as much weight loss and lowering of blood glucose as did semaglutide at a higher dose (14 nmol/kg).
Conclusions: GL0034 is a G protein-biased agonist that shows powerful antidiabetic effects in mice, and may serve as a promising new GLP-1RA for obese patients with type 2 diabetes.
Keywords: GLP-1 analogue; antidiabetic drug; antiobesity drug; beta-cell function; drug development; incretin therapy.
© 2022 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
Vinod Burade and Thennati Rajamannar are employees of Sun Pharmaceuticals, from whom Guy A. Rutter and Alejandra Tomas have received grant funding.
Figures


References
-
- Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the study of diabetes (EASD). Diabetes Care. 2020;43(2):487‐493. doi:10.2337/dci19-0066 - DOI - PMC - PubMed
-
- Zheng SL, Roddick AJ, Aghar‐Jaffar R, et al. Association between use of sodium‐glucose cotransporter 2 inhibitors, glucagon‐like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all‐cause mortality in patients with type 2 diabetes: a systematic review and meta‐analysis. JAMA. 2018;319(15):1580‐1591. doi:10.1001/jama.2018.3024 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

