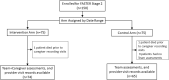
Family-Assisted Severity of Illness Monitoring for Hospitalized Children in Low-Resource Settings-A Two-Arm Interventional Feasibility Study
- PMID: 35676898
- PMCID: PMC9169086
- DOI: 10.3389/fped.2022.804346
Family-Assisted Severity of Illness Monitoring for Hospitalized Children in Low-Resource Settings-A Two-Arm Interventional Feasibility Study
Abstract
Introduction: Pediatric mortality remains unacceptably high in many low-resource settings, with inpatient deaths often associated with delayed recognition of clinical deterioration. The Family-Assisted Severe Febrile Illness ThERapy (FASTER) tool has been developed for caregivers to assist in monitoring their hospitalized children and alert clinicians. This study evaluates feasibility of implementation by caregivers and clinicians.
Methods: Randomized controlled feasibility study at Kenyatta National Hospital, Kenya. Children hospitalized with acute febrile illness with caregivers at the bedside for 24 h were enrolled. Caregivers were trained using the FASTER tool. The primary outcome was the frequency of clinician reassessments between intervention (FASTER) and standard care arms. Poisson regression with random intercept for grouping by patient was used, adjusting for admission pediatric early warning score, age, gender. Secondary outcomes included survey assessments of clinician and caregiver experiences with FASTER.
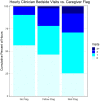
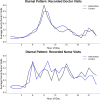
Results: One hundred and fifty patient/caregiver pairs were enrolled, 139 included in the analysis, 74 in the intervention, 65 in the control arm. Patients' median age was 0.9 (range 0.2-10) and 1.1 years (range 0.2-12) in intervention vs. control arms. The most common diagnoses were pneumonia (80[58%]), meningitis (58[38%]) and malaria (34 [24%]). 134 (96%) caregivers were patients' mothers. Clinician visits/hour increased with patients' illness severity in both arms, but without difference in frequency between arms (point estimate for difference -0.9%, p = 0.97). Of the 16 deaths, 8 (four/arm) occurred within 2 days of enrollment. Forty clinicians were surveyed, 33 (82%) reporting that FASTER could improve outcomes of very sick children in low-resource settings; 26 (65%) rating caregivers as able to adequately capture patients' severity of illness. Of 70 caregivers surveyed, 63 (90%) reported that FASTER training was easy to understand; all (100%) agreed that the intervention would improve care of hospitalized children and help identify sick children in their community.
Discussion: We observed no difference in recorded frequency of clinician visits with FASTER monitoring. However, the tool was rated positively by caregivers and clinicians., Implementation appears feasible but requires optimization. These feasibility data may inform a larger trial powered to measure morbidity and mortality outcomes to determine the utility of FASTER in detecting and responding to clinical deterioration in low-resource settings.
Clinical trial registration: ClinicalTrials.gov, identifier: NCT03513861.
Keywords: child health; critical illness; early warning score; global health; low middle income country; low-resource setting; pediatrics.
Copyright © 2022 von Saint Andre-von Arnim, Kumar, Clark, Wilfond, Nguyen, Mutonga, Zimmerman, Oron and Walson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Feasibility of Family-Assisted Severity of Illness Monitoring for Hospitalized Children in Low-Income Settings.Pediatr Crit Care Med. 2021 Feb 1;22(2):e115-e124. doi: 10.1097/PCC.0000000000002582. Pediatr Crit Care Med. 2021. PMID: 33031354
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Efficacy of a Communication-Priming Intervention on Documented Goals-of-Care Discussions in Hospitalized Patients With Serious Illness: A Randomized Clinical Trial.JAMA Netw Open. 2022 Apr 1;5(4):e225088. doi: 10.1001/jamanetworkopen.2022.5088. JAMA Netw Open. 2022. PMID: 35363271 Free PMC article. Clinical Trial.
-
Effect of a Pediatric Early Warning System on All-Cause Mortality in Hospitalized Pediatric Patients: The EPOCH Randomized Clinical Trial.JAMA. 2018 Mar 13;319(10):1002-1012. doi: 10.1001/jama.2018.0948. JAMA. 2018. PMID: 29486493 Free PMC article. Clinical Trial.
-
Clinical Practice Guideline: Tonsillectomy in Children (Update)-Executive Summary.Otolaryngol Head Neck Surg. 2019 Feb;160(2):187-205. doi: 10.1177/0194599818807917. Otolaryngol Head Neck Surg. 2019. PMID: 30921525 Review.
Cited by
-
The role of healthcare providers and caregivers in monitoring critically ill children: a qualitative study in a tertiary hospital, southern Malawi.BMC Health Serv Res. 2024 May 7;24(1):595. doi: 10.1186/s12913-024-11050-8. BMC Health Serv Res. 2024. PMID: 38714998 Free PMC article.
-
Barriers to Pediatric Emergency Care in Low-Resource Settings: A Narrative Review.Sage Open Pediatr. 2025 Apr 28;12:30502225251336861. doi: 10.1177/30502225251336861. eCollection 2025 Jan-Dec. Sage Open Pediatr. 2025. PMID: 40612221 Free PMC article. Review.
-
Family supplemented patient monitoring after surgery (SMARTER): a pilot stepped-wedge cluster-randomised trial.Br J Anaesth. 2024 Oct;133(4):846-852. doi: 10.1016/j.bja.2024.06.027. Epub 2024 Jul 27. Br J Anaesth. 2024. PMID: 39069451 Free PMC article. Clinical Trial.
References
-
- UN Inter-agency Group for Child Mortality Estimation,. Child Mortality Estimates . (2020). Available online at: https://childmortality.org/
-
- GBD 2019 Under-5 Mortality Collaborators . Global, regional, and national progress towards Sustainable Development Goal 3.2 for neonatal and child health: all-cause and cause-specific mortality findings from the Global Burden of Disease Study (2019). Lancet. (2021) 398:870–905. 10.1016/S0140-6736(21)01207-1 - DOI - PMC - PubMed
-
- World Health Organization . Health Workforce Requirements for Universal Health Coverage and the Sustainable Development Goals. Geneva: (2016).
Associated data
LinkOut - more resources
Full Text Sources
Medical