Clinically approved combination immunotherapy: Current status, limitations, and future perspective
- PMID: 35676925
- PMCID: PMC9167882
- DOI: 10.1016/j.crimmu.2022.05.003
Clinically approved combination immunotherapy: Current status, limitations, and future perspective
Abstract
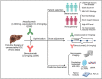
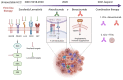
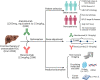
Immune-checkpoint inhibitor-based combination immunotherapy has become a first-line treatment for several major types of cancer including hepatocellular carcinoma (HCC), renal cell carcinoma, lung cancer, cervical cancer, and gastric cancer. Combination immunotherapy counters several immunosuppressive elements in the tumor microenvironment and activates multiple steps of the cancer-immunity cycle. The anti-PD-L1 antibody, atezolizumab, plus the anti-vascular endothelial growth factor antibody, bevacizumab, represents a promising class of combination immunotherapy. This combination has produced unprecedented clinical efficacy in unresectable HCC and become a landmark in HCC therapy. Advanced HCC patients treated with atezolizumab plus bevacizumab demonstrated impressive improvements in multiple clinical endpoints including overall survival, progress-free survival, objective response rate, and patient-reported quality of life when compared to current first-line treatment with sorafenib. However, atezolizumab plus bevacizumab first-line therapy has limitations. First, cancer patients falling into the criteria for the combination therapy may need to be further selected to reap benefits while avoiding some potential pitfalls. Second, the treatment regimen of atezolizumab plus bevacizumab at a fixed dose may require adjustment for optimal normalization of the tumor microenvironment to obtain maximum efficacy and reduce adverse events. Third, utilization of predictive biomarkers is urgently needed to guide the entire treatment process. Here we review the current status of clinically approved combination immunotherapies and the underlying immune mechanisms. We further provide a perspective analysis of the limitations for combination immunotherapies and potential approaches to overcome the limitations.
Keywords: Atezolizumab; Bevacizumab; Biomarker; Combination immunotherapy; Dose; First-line therapy; Gene signature; HCC; Patient selection.
© 2022 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: You-Wen He and Shi-You Li are shareholders of tricision Biotherapeutic Inc.
Figures
References
-
- Batchelor T.T., Sorensen A.G., di Tomaso E., Zhang W.T., Duda D.G., Cohen K.S., Kozak K.R., Cahill D.P., Chen P.J., Zhu M., et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. - PMC - PubMed
-
- Benson A.B., D'Angelica M.I., Abbott D.E., Anaya D.A., Anders R., Are C., Bachini M., Borad M., Brown D., Burgoyne A., et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021;19:541–565. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

