Long Noncoding RNA MIAT Regulates Hyperosmotic Stress-Induced Corneal Epithelial Cell Injury via Inhibiting the Caspase-1-Dependent Pyroptosis and Apoptosis in Dry Eye Disease
- PMID: 35676970
- PMCID: PMC9169976
- DOI: 10.2147/JIR.S361541
Long Noncoding RNA MIAT Regulates Hyperosmotic Stress-Induced Corneal Epithelial Cell Injury via Inhibiting the Caspase-1-Dependent Pyroptosis and Apoptosis in Dry Eye Disease
Abstract
Purpose: The biological role and mechanism of long noncoding RNA (lncRNA) myocardial infarction-associated transcript (MIAT) in dry eye remain to be illustrated. Pyroptosis is a noticeable form of inflammatory activation, which is characteristic of gasdermin D (GSDMD)-driven cell death. The present study was designed to explore the role of MIAT in pyroptosis and apoptosis induced by hyperosmolarity stress (HS) in human corneal epithelial cells (HCECs).
Methods: HCECs were cultured in 70-120 mM hyperosmotic medium for 24 h to create a dry eye model in vitro. The level of the pyroptosis marker GSDMD was measured, and the cell inflammatory response was evaluated by detecting IL-1β and IL-18 levels. Exogenous caspase-1 inhibitor Ac-YVAD-CHO was used. The pyroptosis in HCECs was examined by caspase-1 activity, immunofluorescent staining, and Western blotting. Flow cytometry was performed to test the apoptosis rate of HCECs. Cell migration and proliferation were detected. The expression of the lncRNA MIAT in HCECs was detected by quantitative real-time PCR. MIAT was knocked down by small interfering RNA (siRNA) transfection. The effects of caspase-1 inhibition on pyroptosis, apoptosis, migration, and proliferation were observed.
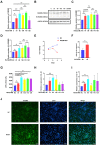
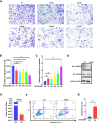
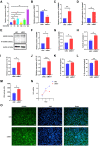
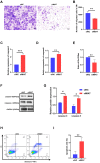
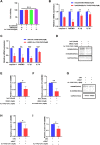
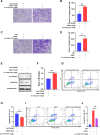
Results: HS promoted pyroptosis in HCECs by elevating caspase-1, GSDMD, and the active cleavage of GSDMD (N-terminal domain, N-GSDMD), and increased the release of IL-1β, IL-18, LDH and the rate of apoptosis, with reduced cell migration. These changes were prevented by the inhibition of caspase-1. The expression of MIAT was significantly increased in HCECs exposed to a hyperosmotic medium. Silencing MIAT increased the expression of GSDMD, caspase-1, and inflammatory chemokines IL-1β and IL-18, and promoted apoptosis while inhibiting migration and proliferation in HCECs.
Conclusion: The lncRNA MIAT is involved in HS-induced pyroptosis and apoptosis and the inflammatory response of HCECs and provides a new understanding of the pathogenesis of dry eye.
Keywords: apoptosis; dry eye; myocardial infarction-associated transcript; pyroptosis.
© 2022 Li et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
IL-1β induced down-regulation of miR-146a-5p promoted pyroptosis and apoptosis of corneal epithelial cell in dry eye disease through targeting STAT3.BMC Ophthalmol. 2024 Mar 29;24(1):144. doi: 10.1186/s12886-024-03396-8. BMC Ophthalmol. 2024. PMID: 38553670 Free PMC article.
-
Calcitriol Alleviates Hyperosmotic Stress-Induced Corneal Epithelial Cell Damage via Inhibiting the NLRP3-ASC-Caspase-1-GSDMD Pyroptosis Pathway in Dry Eye Disease.J Inflamm Res. 2021 Jul 5;14:2955-2962. doi: 10.2147/JIR.S310116. eCollection 2021. J Inflamm Res. 2021. PMID: 34262321 Free PMC article.
-
Caspase 3/GSDME-Mediated Corneal Epithelial Pyroptosis Promotes Dry Eye Disease.Invest Ophthalmol Vis Sci. 2025 Jan 2;66(1):24. doi: 10.1167/iovs.66.1.24. Invest Ophthalmol Vis Sci. 2025. PMID: 39792075 Free PMC article.
-
Dermatophagoides Pteronyssinus 1 (DerP1) May Trigger NLRP3-Mediated Corneal Epithelial Cell Pyroptosis by Elevating Interleukin-33 Expression Levels.Curr Eye Res. 2023 Dec;48(12):1100-1111. doi: 10.1080/02713683.2023.2250583. Epub 2023 Aug 25. Curr Eye Res. 2023. PMID: 37615401
-
Targeting pyroptosis as a preventive and therapeutic approach for stroke.Cell Death Discov. 2023 May 10;9(1):155. doi: 10.1038/s41420-023-01440-y. Cell Death Discov. 2023. PMID: 37165005 Free PMC article. Review.
Cited by
-
The alterations of ocular surface metabolism and the related immunity inflammation in dry eye.Adv Ophthalmol Pract Res. 2024 Aug 14;5(1):1-12. doi: 10.1016/j.aopr.2024.08.003. eCollection 2025 Feb-Mar. Adv Ophthalmol Pract Res. 2024. PMID: 39758836 Free PMC article. Review.
-
Role of Chinese Medicine Monomers in Dry Eye Disease: Breaking the Vicious Cycle of Inflammation.Pharmacol Res Perspect. 2025 Apr;13(2):e70077. doi: 10.1002/prp2.70077. Pharmacol Res Perspect. 2025. PMID: 39979080 Free PMC article. Review.
-
Neutrophil pyroptosis regulates corneal wound healing and post-injury neovascularisation.Clin Transl Med. 2024 Nov;14(11):e1762. doi: 10.1002/ctm2.1762. Clin Transl Med. 2024. PMID: 39496510 Free PMC article.
-
Dopamine Receptor 1 Treatment Promotes Epithelial Repair of Corneal Injury by Inhibiting NOD-Like Receptor Protein 3-Associated Inflammation.Invest Ophthalmol Vis Sci. 2024 Jan 2;65(1):49. doi: 10.1167/iovs.65.1.49. Invest Ophthalmol Vis Sci. 2024. PMID: 38294802 Free PMC article.
-
IL-1β induced down-regulation of miR-146a-5p promoted pyroptosis and apoptosis of corneal epithelial cell in dry eye disease through targeting STAT3.BMC Ophthalmol. 2024 Mar 29;24(1):144. doi: 10.1186/s12886-024-03396-8. BMC Ophthalmol. 2024. PMID: 38553670 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

