Immunogenicity of CAR-T Cell Therapeutics: Evidence, Mechanism and Mitigation
- PMID: 35677038
- PMCID: PMC9169153
- DOI: 10.3389/fimmu.2022.886546
Immunogenicity of CAR-T Cell Therapeutics: Evidence, Mechanism and Mitigation
Abstract
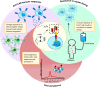
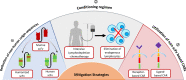
Chimeric antigen receptor T cell (CAR-T) therapy demonstrated remarkable success in long-term remission of cancers and other autoimmune diseases. Currently, six products (Kymriah, Yescarta, Tecartus, Breyanzi, Abecma, and Carvykti) are approved by the US-FDA for treatment of a few hematological malignancies. All the six products are autologous CAR-T cell therapies, where delivery of CAR, which comprises of scFv (single-chain variable fragment) derived from monoclonal antibodies for tumor target antigen recognition is through a lentiviral vector. Although available CAR-T therapies yielded impressive response rates in a large number of patients in comparison to conventional treatment strategies, there are potential challenges in the field which limit their efficacy. One of the major challenges is the induction of humoral and/or cellular immune response in patients elicited due to scFv domain of CAR construct, which is of non-human origin in majority of the commercially available products. Generation of anti-CAR antibodies may lead to the clearance of the therapeutic CAR-T cells, increasing the likelihood of tumor relapse and lower the CAR-T cells efficacy upon reinfusion. These immune responses influence CAR-T cell expansion and persistence, that might affect the overall clinical response. In this review, we will discuss the impact of immunogenicity of the CAR transgene on treatment outcomes. Finally, this review will highlight the mitigation strategies to limit the immunogenic potential of CARs and improve the therapeutic outcome.
Keywords: anti-CAR antibodies; cellular immunity; chimeric antigen receptor; immunogenicity; monoclonal antibodies; persistence; scFv.
Copyright © 2022 Khan, Chowdhury, Karulkar, Jaiswal, Banik, Asija and Purwar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources