MSC-ACE2 Ameliorates Streptococcus uberis-Induced Inflammatory Injury in Mammary Epithelial Cells by Upregulating the IL-10/STAT3/SOCS3 Pathway
- PMID: 35677060
- PMCID: PMC9167935
- DOI: 10.3389/fimmu.2022.870780
MSC-ACE2 Ameliorates Streptococcus uberis-Induced Inflammatory Injury in Mammary Epithelial Cells by Upregulating the IL-10/STAT3/SOCS3 Pathway
Abstract
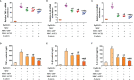
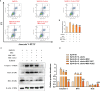
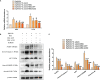
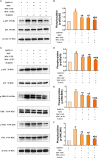
In the dairy industry, Streptococcus uberis (S. uberis) is one of the most important pathogenic bacteria associated with mastitis in milk-producing cows, causing vast economic loss. To date, the only real effective method of treating and preventing streptococcal mastitis is antimicrobial therapy. In many inflammatory diseases, mesenchymal stem cells (MSCs) and angiotensin-converting enzyme 2 (ACE2) play an anti-inflammatory and anti-injurious role. Accordingly, we hypothesized that MSCs overexpressing ACE2 (MSC-ACE2) would ameliorate the inflammatory injury caused by S. uberis in mammary epithelial cells more efficiently than MSC alone. By activating the transcription 3/suppressor of cytokine signaling 3 (IL-10/STAT3/SOCS3) signaling pathway, MSC-ACE2 inhibited the NF-κB, MAPKs, apoptosis, and pyroptosis passways. Moreover, MSC-ACE2 overturned the downregulation of Occludin, Zonula occludens 1 (ZO-1), and Claudin-3 expression levels caused by S. uberis, suggesting that MSC-ACE2 promotes the repair of the blood-milk barrier. MSC-ACE2 demonstrated greater effectiveness than MSC alone, as expected. Based on these results, MSC-ACE2 effectively inhibits EpH4-Ev cell's inflammatory responses induced by S. uberis, and would be an effective therapeutic tool for treating streptococcal mastitis.
Keywords: MSC-ACE2; S. uberis; blood-milk barrier; inflammatory injury; mammary epithelial cells; pyroptosis.
Copyright © 2022 Yan, Zhang, Ji, Wu, Huang, Zhang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



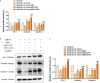
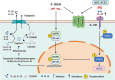
Similar articles
-
Overexpression of angiotensin-converting enzyme 2 contributes to the amelioration of Streptococcus uberis-induced inflammatory injury in mammary epithelial cells.Vet Microbiol. 2022 May;268:109398. doi: 10.1016/j.vetmic.2022.109398. Epub 2022 Mar 19. Vet Microbiol. 2022. PMID: 35339816
-
Mesenchymal Stem Cells Overexpressing ACE2 Favorably Ameliorate LPS-Induced Inflammatory Injury in Mammary Epithelial Cells.Front Immunol. 2022 Jan 14;12:796744. doi: 10.3389/fimmu.2021.796744. eCollection 2021. Front Immunol. 2022. PMID: 35095873 Free PMC article.
-
Mastitis severity induced by two Streptococcus uberis strains is reflected by the mammary immune response in vitro.Schweiz Arch Tierheilkd. 2012 Aug;154(8):317-23. doi: 10.1024/0036-7281/a000355. Schweiz Arch Tierheilkd. 2012. PMID: 22851430
-
Bovine intramammary infection associated immunogenic surface proteins of Streptococcus uberis.Microb Pathog. 2018 Feb;115:304-311. doi: 10.1016/j.micpath.2017.12.046. Epub 2017 Dec 16. Microb Pathog. 2018. PMID: 29258753
-
Potential factors involved in the early pathogenesis of Streptococcus uberis mastitis: a review.Folia Microbiol (Praha). 2021 Aug;66(4):509-523. doi: 10.1007/s12223-021-00879-9. Epub 2021 Jun 3. Folia Microbiol (Praha). 2021. PMID: 34085166 Review.
Cited by
-
Overexpression of the Mas1 gene mitigated LPS-induced inflammatory injury in mammary epithelial cells by inhibiting the NF-κB/MAPKs signaling pathways.Front Vet Sci. 2024 Jul 12;11:1446366. doi: 10.3389/fvets.2024.1446366. eCollection 2024. Front Vet Sci. 2024. PMID: 39071779 Free PMC article.
-
To re-examine the intersection of microglial activation and neuroinflammation in neurodegenerative diseases from the perspective of pyroptosis.Front Aging Neurosci. 2023 Nov 9;15:1284214. doi: 10.3389/fnagi.2023.1284214. eCollection 2023. Front Aging Neurosci. 2023. PMID: 38020781 Free PMC article. Review.
-
Lactiplantibacillus plantarum promotes lactoferrin synthesis and secretion in bovine mammary epithelial cells through STAT3 and AP-1 transcription factor pathways.In Vitro Cell Dev Biol Anim. 2025 Aug 13. doi: 10.1007/s11626-025-01055-w. Online ahead of print. In Vitro Cell Dev Biol Anim. 2025. PMID: 40802133
-
Modulatory Effects of Regulated Cell Death: An Innovative Preventive Approach for the Control of Mastitis.Cells. 2024 Oct 14;13(20):1699. doi: 10.3390/cells13201699. Cells. 2024. PMID: 39451217 Free PMC article. Review.
-
Effects of Zinc (Zn) from Different Sources on Production Performance, Health Status, Antioxidant Properties and Immune Regulation of Dairy Cows in Early Lactation.Vet Sci. 2025 Jun 3;12(6):545. doi: 10.3390/vetsci12060545. Vet Sci. 2025. PMID: 40559782 Free PMC article.
References
-
- Wald R, Baumgartner M, Gutschireiter J, Bazzanella B, Lichtmannsperger K, Wagner M, et al. . Comparison of the Population Structure of Streptococcus Uberis Mastitis Isolates From Austrian Small-Scale Dairy Farms and a Slovakian Large-Scale Farm. J Dairy Sci (2020) 103(2):1820–30. doi: 10.3168/jds.2019-16930 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

