Real-World Treatment Patterns and Clinical Outcomes in Patients With Stage III Non-Small-Cell Lung Cancer: Results of KINDLE-Vietnam Cohort
- PMID: 35677172
- PMCID: PMC9169691
- DOI: 10.3389/fonc.2022.842296
Real-World Treatment Patterns and Clinical Outcomes in Patients With Stage III Non-Small-Cell Lung Cancer: Results of KINDLE-Vietnam Cohort
Abstract
Objective: KINDLE-Vietnam was a part of a real-world KINDLE study with an aim to characterise treatment patterns and clinical outcomes of patients with stage III non-small cell lung cancer (NSCLC).
Materials and methods: Retrospective data from patients diagnosed with stage III NSCLC (American Joint Committee on Cancer, 7th edition) between January 2013 and December 2017 with at least 9 months of follow-up were collected from 2 centres in Vietnam. Descriptive statistics were used to summarise demographics, disease characteristics and treatment modalities. Kaplan-Meier methodology evaluated survival estimates; 2-sided 95% confidence intervals (CIs) were computed. Inferential statistics were used to correlate clinical and treatment variables with median progression-free survival (mPFS) and median overall survival (mOS).
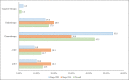
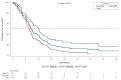
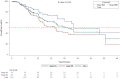
Results: A total of 150 patients (median age: 60 years [range 26-82]) were enrolled; 75.3% were male, 62.0% had smoking history, 56.4% had stage IIIB disease and 62.5% had adenocarcinoma. The majority of the cases (97.3%) were not discussed at a multidisciplinary team meeting. Overall, chemotherapy alone (43.3%), radiotherapy alone (17.0%), sequential chemoradiation (13.5%) and concurrent chemoradiation (12.8%) were preferred as initial therapy. Surgery-based treatment was administered in limited patients (stage IIIA, 10%; stage IIIB, 1.3%). Palliative therapy was the most commonly administered treatment upon relapse in the second-and third-line setting. The mPFS and mOS for the Vietnam cohort were 8.7 months (95% CI, 7.59-9.72) and 25.7 months (95% CI, 19.98-42.61), respectively. The mPFS and mOS for stage IIIA were 11.9 months (95% CI, 8.64-14.95) and 28.2 months (95% CI, 24.15-not-calculable) and for stage IIIB were 7.8 months (95% CI, 6.64-8.71) and 20.0 months (95% CI, 13.01-42.61).
Conclusions: KINDLE-Vietnam offers insights into the clinical findings of stage III NSCLC. There is a high unmet need for identifying patients in the early stages of NSCLC. Strategies for improving clinical outcomes in this patient population include physician education, multidisciplinary management and catering to increased access to novel agents like immunotherapy and targeted therapy.
Keywords: chemoradiotherapy; chemotherapy; lung cancer; sequential chemoradiation; stage III NSCLC.
Copyright © 2022 Van Dao, Diep, Le Phuong, Huggenberger and Kumar.
Conflict of interest statement
Authors RH, AK and TP are employed by the company AstraZeneca. The authors declare that the KINDLE study and this manuscript writing were funded by AstraZeneca. The funder had the following involvement with the study: study design, analysis, interpretation of data, and the writing of this article.
Figures
Similar articles
-
Real-World Treatment Patterns and Clinical Outcomes in Patients With Stage III NSCLC: Results of KINDLE, a Multicountry Observational Study.J Thorac Oncol. 2021 Oct;16(10):1733-1744. doi: 10.1016/j.jtho.2021.05.003. Epub 2021 May 26. J Thorac Oncol. 2021. PMID: 34051381
-
Real-world clinical practice and outcomes in treating stage III non-small cell lung cancer: KINDLE-Asia subset.Front Oncol. 2023 Mar 27;13:1117348. doi: 10.3389/fonc.2023.1117348. eCollection 2023. Front Oncol. 2023. PMID: 37051534 Free PMC article.
-
Real-world KINDLE-Latin America subset data on treatment patterns and clinical outcomes in patients with stage III non-small-cell lung cancer.Cancer Med. 2023 Jan;12(2):1247-1259. doi: 10.1002/cam4.4990. Epub 2022 Jul 4. Cancer Med. 2023. PMID: 35789068 Free PMC article.
-
Radiotherapy and chemotherapy in locally advanced non-small cell lung cancer: preclinical and early clinical data.Hematol Oncol Clin North Am. 2004 Feb;18(1):41-53. doi: 10.1016/s0889-8588(03)00138-2. Hematol Oncol Clin North Am. 2004. PMID: 15005280 Review.
-
Sequential combined modality therapy for stage III non-small cell lung cancer.Hematol Oncol Clin North Am. 1990 Dec;4(6):1133-42. Hematol Oncol Clin North Am. 1990. PMID: 1962780 Review.
Cited by
-
Treatment patterns and survival analysis in patients with unresectable stage III EGFR-mutated non-small cell lung cancer.Aging (Albany NY). 2024 Jan 11;16(1):857-871. doi: 10.18632/aging.205425. Epub 2024 Jan 11. Aging (Albany NY). 2024. PMID: 38214678 Free PMC article.
-
Prevalence of EGFR Mutations in Vietnamese Patients with Resected Early Stage Non-Small Cell Lung Cancer: EARLY-EGFR Study.Lung Cancer (Auckl). 2025 Apr 21;16:39-49. doi: 10.2147/LCTT.S494554. eCollection 2025. Lung Cancer (Auckl). 2025. PMID: 40291011 Free PMC article.
-
Overview of approaches to estimate real-world disease progression in lung cancer.JNCI Cancer Spectr. 2023 Oct 31;7(6):pkad074. doi: 10.1093/jncics/pkad074. JNCI Cancer Spectr. 2023. PMID: 37738580 Free PMC article. Review.
-
Assessing the clinical, humanistic, and economic impact of early cancer diagnosis: a systematic literature review.Front Oncol. 2025 Mar 19;15:1546447. doi: 10.3389/fonc.2025.1546447. eCollection 2025. Front Oncol. 2025. PMID: 40177242 Free PMC article.
References
-
- Globocan . WHO South-East Asia Region. In: International Agency for Research on Cancer. France: Publisher International Agency for Research on Cancer City; (2020). Available at: https://gco.iarc.fr/today/data/factsheets/populations/995-who-south-east....
-
- Globocan . Vietnam Factsheet. In: International Agency for Research on Cancer. France: Publisher International Agency for Research on Cancer City; (2020). Available at: https://gco.iarc.fr/today/data/factsheets/populations/704-viet-nam-fact-....
LinkOut - more resources
Full Text Sources

