AgNPs Aggravated Hepatic Steatosis, Inflammation, Oxidative Stress, and Epigenetic Changes in Mice With NAFLD Induced by HFD
- PMID: 35677306
- PMCID: PMC9169095
- DOI: 10.3389/fbioe.2022.912178
AgNPs Aggravated Hepatic Steatosis, Inflammation, Oxidative Stress, and Epigenetic Changes in Mice With NAFLD Induced by HFD
Abstract
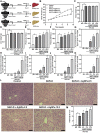
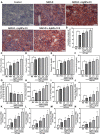
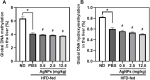
The recent development of silver nanoparticles (AgNPs) has sparked increased interest in biomedical and pharmaceutical applications, leading to the possibility of human exposure. The liver is the primary target organ in the metabolism and transport of nanoparticles. Non-alcoholic fatty liver disease (NAFLD) is the most common and leading cause of hepatic metabolic syndrome with approximately 15% of patients will develop into non-alcoholic steatohepatitis, fibrosis, cirrhosis, and eventually hepatocellular carcinoma. Thus, the potential hepatotoxicity of AgNPs on NAFLD development and progression should be of great concern. Herein, we explored the potential hepatic effect of a single intravenously injected dose of 0.5, 2.5, and 12.5 mg/kg BW on the liver function of high-fat-diet (HFD)-fed mice for 7 days. AgNP treatment increased serum levels of alanine aminotransferase, aspartate transaminase, triglycerides and cholesterols, the number of lipid droplets, and the contents of triglycerides and cholesterols in NAFLD mice livers compared to HFD-fed mice. The mechanism of AgNP-induced worsen hepatotoxicity in mice is associated with hyperactivation of SREBP-1c-mediated de novo lipogenesis and liver inflammation. Additionally, HFD-fed mice treated with AgNPs had significantly higher oxidative damage and lower global DNA methylation and DNA hydroxymethylation than NAFLD mice. This study suggests that AgNP treatment exacerbated HFD-induced hepatic steatosis, liver inflammation, oxidative stress, and epigenetic changes in mice, which is relevant to the risk of AgNP exposure on NAFLD development and progression.
Keywords: global DNA methylation; hepatic steatosis; hepatotoxicity; liver inflammation; non-alcoholic fatty liver disease; silver nanoparticles.
Copyright © 2022 Wen, Li, Lin, Li, Song and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Tetrahydropalmatine ameliorates hepatic steatosis in nonalcoholic fatty liver disease by switching lipid metabolism via AMPK-SREBP-1c-Sirt1 signaling axis.Phytomedicine. 2023 Oct;119:155005. doi: 10.1016/j.phymed.2023.155005. Epub 2023 Aug 5. Phytomedicine. 2023. PMID: 37562090
-
Targeting DUSP7 signaling alleviates hepatic steatosis, inflammation and oxidative stress in high fat diet (HFD)-fed mice via suppression of TAK1.Free Radic Biol Med. 2020 Jun;153:140-158. doi: 10.1016/j.freeradbiomed.2020.04.009. Epub 2020 Apr 18. Free Radic Biol Med. 2020. PMID: 32311490
-
Intermittent restraint-induced sympathetic activation attenuates hepatic steatosis and inflammation in a high-fat diet-fed mouse model.Am J Physiol Gastrointest Liver Physiol. 2019 Dec 1;317(6):G811-G823. doi: 10.1152/ajpgi.00047.2019. Epub 2019 Oct 11. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31604029
-
Non-Alcoholic Fatty Liver Disease.Adv Exp Med Biol. 2017;960:443-467. doi: 10.1007/978-3-319-48382-5_19. Adv Exp Med Biol. 2017. PMID: 28585211 Review.
-
Connection of Nicotine to Diet-Induced Obesity and Non-Alcoholic Fatty Liver Disease: Cellular and Mechanistic Insights.Front Endocrinol (Lausanne). 2017 Feb 10;8:23. doi: 10.3389/fendo.2017.00023. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28239368 Free PMC article. Review.
Cited by
-
In Vivo Pro-Inflammatory Effects of Silver Nanoparticles on the Colon Depend on Time and Route of Exposure.Int J Mol Sci. 2024 Apr 30;25(9):4879. doi: 10.3390/ijms25094879. Int J Mol Sci. 2024. PMID: 38732098 Free PMC article.
-
Increased DNMT1 Involvement in the Activation of LO2 Cell Death Induced by Silver Nanoparticles via Promoting TFEB-Dependent Autophagy.Toxics. 2023 Sep 4;11(9):751. doi: 10.3390/toxics11090751. Toxics. 2023. PMID: 37755761 Free PMC article.
-
Phytonanotherapy for the Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease.Int J Mol Sci. 2024 May 21;25(11):5571. doi: 10.3390/ijms25115571. Int J Mol Sci. 2024. PMID: 38891759 Free PMC article. Review.
-
Diet, oxidative stress and MAFLD: a mini review.Front Nutr. 2025 Mar 4;12:1539578. doi: 10.3389/fnut.2025.1539578. eCollection 2025. Front Nutr. 2025. PMID: 40104813 Free PMC article. Review.
-
Evaluation of the Safety and Efficacy of Curcumin-Synthesized Silver Nanoparticles in Rats Exposed to Chlorpyrifos During Puberty Development.Curr Mol Med. 2024;24(11):1437-1444. doi: 10.2174/0115665240259497231020070225. Curr Mol Med. 2024. PMID: 39420715
References
-
- Chen H., Zhou S., Zhu M., Wang B., Chen W., Zheng L., et al. (2021). Gold Nanoparticles Modified with Polyethyleneimine Disturbed the Activity of Drug-Metabolic Enzymes and Induced Inflammation-Mediated Liver Injury in Mice. Front. Pharmacol. 12, 706791. 10.3389/fphar.2021.706791 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

