Recent Advances in Aptasensors For Rapid and Sensitive Detection of Staphylococcus Aureus
- PMID: 35677308
- PMCID: PMC9169243
- DOI: 10.3389/fbioe.2022.889431
Recent Advances in Aptasensors For Rapid and Sensitive Detection of Staphylococcus Aureus
Abstract
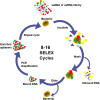
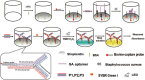
The infection of Staphylococcus aureus (S.aureus) and the spread of drug-resistant bacteria pose a serious threat to global public health. Therefore, timely, rapid and accurate detection of S. aureus is of great significance for food safety, environmental monitoring, clinical diagnosis and treatment, and prevention of drug-resistant bacteria dissemination. Traditional S. aureus detection methods such as culture identification, ELISA, PCR, MALDI-TOF-MS and sequencing, etc., have good sensitivity and specificity, but they are complex to operate, requiring professionals and expensive and complex machines. Therefore, it is still challenging to develop a fast, simple, low-cost, specific and sensitive S. aureus detection method. Recent studies have demonstrated that fast, specific, low-cost, low sample volume, automated, and portable aptasensors have been widely used for S. aureus detection and have been proposed as the most attractive alternatives to their traditional detection methods. In this review, recent advances of aptasensors based on different transducer (optical and electrochemical) for S. aureus detection have been discussed in details. Furthermore, the applications of aptasensors in point-of-care testing (POCT) have also been discussed. More and more aptasensors are combined with nanomaterials as efficient transducers and amplifiers, which appears to be the development trend in aptasensors. Finally, some significant challenges for the development and application of aptasensors are outlined.
Keywords: POCT; Staphylococcus aureus; aptasensor; electrochemical biosensor; nanomaterials; optical biosensor.
Copyright © 2022 Chen, Lai, Zhang and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Banerjee J. (2010). Antibodies Are Challenged. Indian J. Med. Sci. 64, 144–147. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

