VE607 stabilizes SARS-CoV-2 Spike in the "RBD-up" conformation and inhibits viral entry
- PMID: 35677392
- PMCID: PMC9164512
- DOI: 10.1016/j.isci.2022.104528
VE607 stabilizes SARS-CoV-2 Spike in the "RBD-up" conformation and inhibits viral entry
Abstract
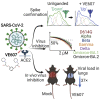
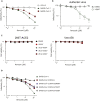
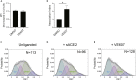
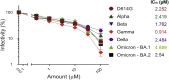
SARS-CoV-2 infection of host cells starts by binding the Spike glycoprotein (S) to the ACE2 receptor. The S-ACE2 interaction is a potential target for therapies against COVID-19 as demonstrated by the development of immunotherapies blocking this interaction. VE607 - a commercially available compound composed of three stereoisomers - was described as an inhibitor of SARS-CoV-1. Here, we show that VE607 broadly inhibits pseudoviral particles bearing the Spike from major VOCs (D614G, Alpha, Beta, Gamma, Delta, Omicron - BA.1, and BA.2) as well as authentic SARS-CoV-2 at low micromolar concentrations. In silico docking, mutational analysis, and smFRET revealed that VE607 binds to the receptor binding domain (RBD)-ACE2 interface and stabilizes RBD in its "up" conformation. Prophylactic treatment with VE607 did not prevent SARS-CoV-2-induced mortality in K18-hACE2 mice, but it did reduce viral replication in the lungs by 37-fold. Thus, VE607 is an interesting lead for drug development for the treatment of SARS-CoV-2 infection.
Keywords: Drugs; Virology.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Update of
-
VE607 Stabilizes SARS-CoV-2 Spike In the "RBD-up" Conformation and Inhibits Viral Entry.bioRxiv [Preprint]. 2022 Feb 22:2022.02.03.479007. doi: 10.1101/2022.02.03.479007. bioRxiv. 2022. Update in: iScience. 2022 Jul 15;25(7):104528. doi: 10.1016/j.isci.2022.104528. PMID: 35233570 Free PMC article. Updated. Preprint.
Similar articles
-
VE607 Stabilizes SARS-CoV-2 Spike In the "RBD-up" Conformation and Inhibits Viral Entry.bioRxiv [Preprint]. 2022 Feb 22:2022.02.03.479007. doi: 10.1101/2022.02.03.479007. bioRxiv. 2022. Update in: iScience. 2022 Jul 15;25(7):104528. doi: 10.1016/j.isci.2022.104528. PMID: 35233570 Free PMC article. Updated. Preprint.
-
Inhibition of S-protein RBD and hACE2 Interaction for Control of SARSCoV- 2 Infection (COVID-19).Mini Rev Med Chem. 2021;21(6):689-703. doi: 10.2174/1389557520666201117111259. Mini Rev Med Chem. 2021. PMID: 33208074 Review.
-
Mutational landscape and in silico structure models of SARS-CoV-2 spike receptor binding domain reveal key molecular determinants for virus-host interaction.BMC Mol Cell Biol. 2022 Jan 7;23(1):2. doi: 10.1186/s12860-021-00403-4. BMC Mol Cell Biol. 2022. PMID: 34991443 Free PMC article.
-
Understanding the Driving Forces That Trigger Mutations in SARS-CoV-2: Mutational Energetics and the Role of Arginine Blockers in COVID-19 Therapy.Viruses. 2022 May 11;14(5):1029. doi: 10.3390/v14051029. Viruses. 2022. PMID: 35632769 Free PMC article.
-
Therapeutic potential of green tea catechin, (-)-epigallocatechin-3-O-gallate (EGCG) in SARS-CoV-2 infection: Major interactions with host/virus proteases.Phytomed Plus. 2023 Feb;3(1):100402. doi: 10.1016/j.phyplu.2022.100402. Epub 2022 Dec 30. Phytomed Plus. 2023. PMID: 36597465 Free PMC article. Review.
Cited by
-
Temperature-dependent Spike-ACE2 interaction of Omicron subvariants is associated with viral transmission.mBio. 2024 Aug 14;15(8):e0090724. doi: 10.1128/mbio.00907-24. Epub 2024 Jul 2. mBio. 2024. PMID: 38953636 Free PMC article.
-
Drug repurposing of argatroban, glimepiride and ranolazine shows anti-SARS-CoV-2 activity via diverse mechanisms.Heliyon. 2025 Jan 10;11(3):e41894. doi: 10.1016/j.heliyon.2025.e41894. eCollection 2025 Feb 15. Heliyon. 2025. PMID: 39968139 Free PMC article.
-
Temperature Influences the Interaction between SARS-CoV-2 Spike from Omicron Subvariants and Human ACE2.Viruses. 2022 Sep 30;14(10):2178. doi: 10.3390/v14102178. Viruses. 2022. PMID: 36298733 Free PMC article.
-
Humoral Responses Elicited by SARS-CoV-2 mRNA Vaccine in People Living with HIV.Viruses. 2023 Sep 26;15(10):2004. doi: 10.3390/v15102004. Viruses. 2023. PMID: 37896781 Free PMC article.
-
Identification of broad, potent antibodies to functionally constrained regions of SARS-CoV-2 spike following a breakthrough infection.Proc Natl Acad Sci U S A. 2023 Jun 6;120(23):e2220948120. doi: 10.1073/pnas.2220948120. Epub 2023 May 30. Proc Natl Acad Sci U S A. 2023. PMID: 37253011 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

