The Antithrombotic Effect of Recombinant Neorudin on Thrombi
- PMID: 35677424
- PMCID: PMC9169676
- DOI: 10.2147/DDDT.S353088
The Antithrombotic Effect of Recombinant Neorudin on Thrombi
Abstract
Introduction: Recombinant neorudin (EPR-hirudin, EH) was developed through the addition of an EPR (Glu-Pro-Arg) peptide to the amino terminus of hirudin, which can be recognized and cut by coagulation factors XIa (FXIa) and/or Xa (FXa). In this study, the low-bleeding antithrombotic effects of EH were evaluated utilizing experimental models of thrombosis in rabbits and rats to provide a test basis for clinical trials.
Methods: The bleeding risks of EH and hirudin were first compared in mice by the tail-clipping method, and then the antithrombotic activity of EH was investigated in a rabbit model of arteriovenous bypass thrombosis and a rat model of thrombotic cerebral infarction.
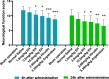
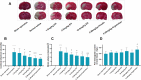
Results: In mice, intravenous administration of EH at 1.5 mg/kg and 3 mg/kg did not affect the bleeding time compared with normal saline, while the administration of hirudin at 1.5 mg/kg prolonged the bleeding time by over 3 times the administration of normal saline. Furthermore, intravenous administration of EH had a significant dose-dependent inhibitory effect on the formation and development of arteriovenous bypass thrombosis and thrombotic cerebral infarction. Compared with an equimolar dose of hirudin, the antithrombotic effect of EH was similar, while the bleeding side effects were significantly attenuated. Moreover, when the antithrombotic effects were similar, EH had a shorter bleeding time and was associated with less bleeding than low molecular weight heparin (LMWH). EH had a therapeutic effect on thrombotic cerebral infarction without increasing the occurrence of cerebral hemorrhage.
Conclusion: The findings from the preclinical animal models used in this study showed that EH could not only effectively inhibit thrombus formation but also reduce the risk of bleeding.
Keywords: antithrombotic effect; bleeding; hirudin; recombinant neorudin.
© 2022 Liu et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Recombinant Neorudin for the Prevention of Deep-Vein Thrombosis After Spinal-Cord Injury.Drug Des Devel Ther. 2023 Aug 23;17:2523-2535. doi: 10.2147/DDDT.S408078. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37641688 Free PMC article.
-
Inhibitory role of recombinant neorudin on canine coronary artery thrombosis.Pharmacol Res Perspect. 2022 Jun;10(3):e00956. doi: 10.1002/prp2.956. Pharmacol Res Perspect. 2022. PMID: 35505637 Free PMC article.
-
Additive effect of the combined administration of low molecular weight heparin and recombinant hirudin on thrombus growth in a rabbit jugular vein thrombosis model.Thromb Haemost. 1994 Sep;72(3):377-80. Thromb Haemost. 1994. PMID: 7855787
-
Hirudin versus heparin and low-molecular-weight heparin: and the winner is..J Lab Clin Med. 1998 Sep;132(3):171-4. doi: 10.1016/s0022-2143(98)90164-0. J Lab Clin Med. 1998. PMID: 9735921 Review. No abstract available.
-
Anticoagulant and antithrombotic actions of recombinant hirudin.Semin Thromb Hemost. 1991 Apr;17(2):130-6. doi: 10.1055/s-2007-1002601. Semin Thromb Hemost. 1991. PMID: 1837615 Review. No abstract available.
Cited by
-
Recombinant Neorudin for the Prevention of Deep-Vein Thrombosis After Spinal-Cord Injury.Drug Des Devel Ther. 2023 Aug 23;17:2523-2535. doi: 10.2147/DDDT.S408078. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37641688 Free PMC article.
-
Recombinant neorudin and its active metabolite hirudin: the fate in vivo of a novel anticoagulant drug.Front Pharmacol. 2024 Sep 17;15:1443475. doi: 10.3389/fphar.2024.1443475. eCollection 2024. Front Pharmacol. 2024. PMID: 39355775 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

