Impedance-Based Phenotypic Readout of Transporter Function: A Case for Glutamate Transporters
- PMID: 35677430
- PMCID: PMC9169222
- DOI: 10.3389/fphar.2022.872335
Impedance-Based Phenotypic Readout of Transporter Function: A Case for Glutamate Transporters
Abstract
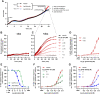
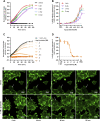
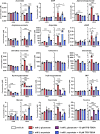
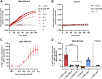
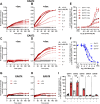
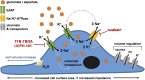
Excitatory amino acid transporters (EAAT/SLC1) mediate Na+-dependent uptake of extracellular glutamate and are potential drug targets for neurological disorders. Conventional methods to assess glutamate transport in vitro are based on radiolabels, fluorescent dyes or electrophysiology, which potentially compromise the cell's physiology and are generally less suited for primary drug screens. Here, we describe a novel label-free method to assess human EAAT function in living cells, i.e., without the use of chemical modifications to the substrate or cellular environment. In adherent HEK293 cells overexpressing EAAT1, stimulation with glutamate or aspartate induced cell spreading, which was detected in real-time using an impedance-based biosensor. This change in cell morphology was prevented in the presence of the Na+/K+-ATPase inhibitor ouabain and EAAT inhibitors, which suggests the substrate-induced response was ion-dependent and transporter-specific. A mechanistic explanation for the phenotypic response was substantiated by actin cytoskeleton remodeling and changes in the intracellular levels of the osmolyte taurine, which suggests that the response involves cell swelling. In addition, substrate-induced cellular responses were observed for cells expressing other EAAT subtypes, as well as in a breast cancer cell line (MDA-MB-468) with endogenous EAAT1 expression. These findings allowed the development of a label-free high-throughput screening assay, which could be beneficial in early drug discovery for EAATs and holds potential for the study of other transport proteins that modulate cell shape.
Keywords: EAAT; cell swelling; glutamate transporter; impedance; label-free; phenotypic assay; solute carrier.
Copyright © 2022 Sijben, Dall’ Acqua, Liu, Jarret, Christodoulaki, Onstein, Wolf, Verburgt, Le Dévédec, Wiedmer, Superti-Furga, IJzerman and Heitman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Abrahamsen B., Schneider N., Erichsen M. N., Huynh T. H., Fahlke C., Bunch L., et al. (2013). Allosteric Modulation of an Excitatory Amino Acid Transporter: the Subtype-Selective Inhibitor UCPH-101 Exerts Sustained Inhibition of EAAT1 through an Intramonomeric Site in the Trimerization Domain. J. Neurosci. 33, 1068–1087. 10.1523/JNEUROSCI.3396-12.2013 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

