Treatment of Open-Angle Glaucoma and Ocular Hypertension with the Fixed-Dose Combination of Preservative-Free Tafluprost/Timolol: Clinical Outcomes from Ophthalmology Clinics in Italy
- PMID: 35677639
- PMCID: PMC9167838
- DOI: 10.2147/OPTH.S364880
Treatment of Open-Angle Glaucoma and Ocular Hypertension with the Fixed-Dose Combination of Preservative-Free Tafluprost/Timolol: Clinical Outcomes from Ophthalmology Clinics in Italy
Abstract
Introduction: The VISIONARY study examined the intraocular pressure (IOP)-lowering efficacy and tolerability of the preservative-free fixed-dose combination of tafluprost (0.0015%) and timolol (0.5%) (PF tafluprost/timolol FC) in a real-world setting. The country-level data reported herein comprise the largest and first observational study of PF tafluprost/timolol FC therapy in Italy.
Methods: An observational, multicenter, prospective study included adult Italian patients with open-angle glaucoma (OAG) or ocular hypertension (OHT) demonstrating insufficient response or poor tolerability with topical prostaglandin analogue (PGA) or beta-blocker monotherapy. Treatment was switched to PF tafluprost/timolol FC therapy at baseline. Primary endpoint was the absolute mean IOP change from baseline at Month 6. Exploratory and safety endpoints included change in IOP at Weeks 4 and 12, ocular signs, symptom severity and reporting of adverse events (AEs).
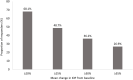
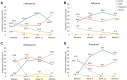
Results: Overall, 160 OAG/OHT patients were included. Mean ± standard deviation IOP was reduced from 19.6 ± 3.6 mmHg at baseline to 14.5 ± 2.6 mmHg at Month 6 (reduction of 5.1 ± 3.7 mmHg; 24.1%; p < 0.0001). IOP reduction was also statistically significant at Week 4 (23.1%; p < 0.0001) and Week 12 (24.7%; p < 0.0001). Based on data cutoff values for mean IOP change of ≥20%, ≥25%, ≥30% and ≥35%, respective Month 6 responder rates were 68.1%, 48.7%, 36.2% and 26.9%. Most ocular signs and symptoms were significantly reduced in severity from baseline at Month 6. Two non-serious and mild AEs were reported during the study period, among which, one AE was treatment-related (eyelash growth). .
Conclusion: Italian OAG and OHT patients demonstrated a significant IOP reduction from baseline at Week 4 that was maintained over a 6-month period following a switch from topical PGA or beta-blocker monotherapy to PF tafluprost/timolol FC therapy. Severity of most ocular signs and symptoms was significantly reduced during the study period, and PF tafluprost/timolol FC was generally well tolerated.
Keywords: PF tafluprost/timolol FC; fixed-dose combination therapy; intraocular pressure; ocular hypertension; preservative-free topical medication; primary open-angle glaucoma.
© 2022 Oddone et al.
Conflict of interest statement
Francesco Oddone has received consultancy fees from Santen, Allergan, Sooft, Thea, Omikron Italia, Centervue and Novartis. Vincenzo Scorcia has no relevant financial disclosures. Michele Iester has received consultancy fees from SIFI and Thea, speaker grant from Santen, Omikron Italia and Thea. Dario Sisto has no relevant financial disclosures. Stefano De Cilla has no relevant financial disclosures. Paolo Bettin has not received any fee from any company in the last two years. Carlo Cagini has no relevant financial disclosures. Michele Figus has no relevant financial disclosures. Giorgio Marchini has no relevant financial disclosures. Luca Rossetti received fees from Aerie, Alcon, Allergan, Centervue, Novartis, NTC, Omikron. Gemma Rossi has no relevant financial disclosures. Tommaso Salgarello received no specific funding for this work. Gian Luca Scuderi has received consultancy fees from Santen, Allergan, Sooft, Omikron Italy. Giovanni Staurenghi has received consultant/advisor fees from Heidelberg Engineering, Centervue, Apellis, Allergan, Astellas, Bayer, Boehringer, Genentech, Iveric, Novartis, Roche and Chengdu Kanghong Biotechnology Co, Kyoto Drug Discovery & Development Co; grant support from Heidelberg Engineering, Optos, Optovue, Quantel Medical, Centervue, Carl Zeiss Meditec, Nidek and Topcon; lecture fees from Heidelberg Engineering, Nidek, Bayer, Novartis and Roche; patents/royalty from Ocular Instruments. The authors report no other conflicts of interest in this work.
Figures
References
-
- European Glaucoma Society. Terminology and guidelines for glaucoma 5th edition; 2020. Available from: https://www.eugs.org/eng/egs_guidelines_download.asp. Accessed January 20, 2022. - PubMed
LinkOut - more resources
Full Text Sources

