Experimental Models to Study the Pathogenesis of Malaria-Associated Acute Respiratory Distress Syndrome
- PMID: 35677654
- PMCID: PMC9168995
- DOI: 10.3389/fcimb.2022.899581
Experimental Models to Study the Pathogenesis of Malaria-Associated Acute Respiratory Distress Syndrome
Abstract
Malaria-associated acute respiratory distress syndrome (MA-ARDS) is increasingly gaining recognition as a severe malaria complication because of poor prognostic outcomes, high lethality rate, and limited therapeutic interventions. Unfortunately, invasive clinical studies are challenging to conduct and yields insufficient mechanistic insights. These limitations have led to the development of suitable MA-ARDS experimental mouse models. In patients and mice, MA-ARDS is characterized by edematous lung, along with marked infiltration of inflammatory cells and damage of the alveolar-capillary barriers. Although, the pathogenic pathways have yet to be fully understood, the use of different experimental mouse models is fundamental in the identification of mediators of pulmonary vascular damage. In this review, we discuss the current knowledge on endothelial activation, leukocyte recruitment, leukocyte induced-endothelial dysfunction, and other important findings, to better understand the pathogenesis pathways leading to endothelial pulmonary barrier lesions and increased vascular permeability. We also discuss how the advances in imaging techniques can contribute to a better understanding of the lung lesions induced during MA-ARDS, and how it could aid to monitor MA-ARDS severity.
Keywords: ARDS; Plasmodium berghei; endothelial dysfunction; mouse; pulmonary vascular damage; vascular permeability.
Copyright © 2022 Nguee, Júnior, Epiphanio, Rénia and Claser.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
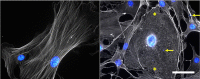
Figures
References
-
- Ampawong S., Chaisri U., Viriyavejakul P., Prapansilp P., Grau G. E., Turner G. D., et al. (2015). A Potential Role for Interleukin-33 and Gamma-Epithelium Sodium Channel in the Pathogenesis of Human Malaria Associated Lung Injury. Malar. J. 14, 389. doi: 10.1186/s12936-015-0922-x - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

