Chronic Stress and Ovulatory Dysfunction: Implications in Times of COVID-19
- PMID: 35677754
- PMCID: PMC9168655
- DOI: 10.3389/fgwh.2022.866104
Chronic Stress and Ovulatory Dysfunction: Implications in Times of COVID-19
Abstract
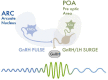
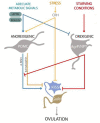
Stress is known to be associated with adverse health outcomes. The COVID-19 pandemic and its associated lockdowns are examples of chronic stressors. Lockdown measures inadvertently caused significant psychological distress and became a powerful source of anxiety/stress, sleep disturbances, nutritional changes and weight gain. Stress is known to impact women's health specifically, through hypothalamic-pituitary-gonadal (HPG) axis dysfunction and resultant ovulatory dysfunction. Such dysfunction may manifest in menstrual irregularities and/or infertility due to hypothalamic hypogonadism. Here, we review the key physiological mediators of stress and associated ovulatory dysfunction. The kisspeptinergic system is comprised of sets of neurons located in the hypothalamus, the rostral periventricular region of the third ventricle (RP3V) and the arcuate nucleus (ARC). This system links nutrition, reproductive signals and stress. It plays a key role in the function of the HPG axis. During chronic stress, the kisspeptinergic system affects the HPG axis, GnRH pulsatility, and, therefore, ovulation. Leptin, insulin and corticotrophin-releasing hormone (CRH) are thought to be additional key modulators in the behavioral responses to chronic stress and may contribute to stress-related ovulatory dysfunction. This mini-review also summarizes and appraises the available evidence on the negative impact of chronic stress as a result of the COVID-19 pandemic lockdowns. It proposes physiological mechanisms to explain the observed effects on women's reproductive health and well-being. The review suggests areas for future research.
Keywords: anxiety; kisspeptin; menstrual cycle; obesity; ovulation; stress.
Copyright © 2022 Vigil, Meléndez, Soto, Petkovic, Bernal and Molina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

