Void-free 3D Bioprinting for In-situ Endothelialization and Microfluidic Perfusion
- PMID: 35677899
- PMCID: PMC7612826
- DOI: 10.1002/adfm.201909009
Void-free 3D Bioprinting for In-situ Endothelialization and Microfluidic Perfusion
Abstract
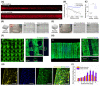
Two major challenges of 3D bioprinting are the retention of structural fidelity and efficient endothelialization for tissue vascularization. We address both of these issues by introducing a versatile 3D bioprinting strategy, in which a templating bioink is deposited layer-by-layer alongside a matrix bioink to establish void-free multimaterial structures. After crosslinking the matrix phase, the templating phase is sacrificed to create a well-defined 3D network of interconnected tubular channels. This void-free 3D printing (VF-3DP) approach circumvents the traditional concerns of structural collapse, deformation and oxygen inhibition, moreover, it can be readily used to print materials that are widely considered "unprintable". By pre-loading endothelial cells into the templating bioink, the inner surface of the channels can be efficiently cellularized with a confluent endothelial layer. This in-situ endothelialization method can be used to produce endothelium with a far greater uniformity than can be achieved using the conventional post-seeding approach. This VF-3DP approach can also be extended beyond tissue fabrication and towards customized hydrogel-based microfluidics and self-supported perfusable hydrogel constructs.
Keywords: bioprinting; endothelialization; hydrogels; microfluidics; printability.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
