High-Pressure Microfluidics for Ultra-Fast Microbial Phenotyping
- PMID: 35677901
- PMCID: PMC9168469
- DOI: 10.3389/fmicb.2022.866681
High-Pressure Microfluidics for Ultra-Fast Microbial Phenotyping
Abstract
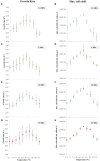
Here, we present a novel methodology based on high-pressure microfluidics to rapidly perform temperature-based phenotyping of microbial strains from deep-sea environments. The main advantage concerns the multiple on-chip temperature conditions that can be achieved in a single experiment at pressures representative of the deep-sea, overcoming the conventional limitations of large-scale batch metal reactors to conduct fast screening investigations. We monitored the growth of the model strain Thermococcus barophilus over 40 temperature and pressure conditions, without any decompression, in only 1 week, whereas it takes weeks or months with conventional approaches. The results are later compared with data from the literature. An additional example is also shown for a hydrogenotrophic methanogen strain (Methanothermococcus thermolithotrophicus), demonstrating the robustness of the methodology. These microfluidic tools can be used in laboratories to accelerate characterizations of new isolated species, changing the widely accepted paradigm that high-pressure microbiology experiments are time-consuming.
Keywords: deep-sea microorganisms; fast screening; high-pressure microfluidics; phenotyping; real time investigations.
Copyright © 2022 Cario, Larzillière, Nguyen, Alain and Marre.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Bao C., Gai Y., Lou K., Jiang C., Ye S. (2010). High-hydrostatic-pressure optical chamber system for cultivation and microscopic observation of deep-sea organisms. Aquat. Biol. 11, 157–162. doi: 10.3354/ab00303 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

