Recent advances and clinical pharmacology aspects of Chimeric Antigen Receptor (CAR) T-cellular therapy development
- PMID: 35677992
- PMCID: PMC9468561
- DOI: 10.1111/cts.13349
Recent advances and clinical pharmacology aspects of Chimeric Antigen Receptor (CAR) T-cellular therapy development
Abstract
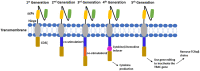
Advances in immuno-oncology have provided a variety of novel therapeutics that harness the innate immune system to identify and destroy neoplastic cells. It is noteworthy that acceptable safety profiles accompany the development of these targeted therapies, which result in efficacious cancer treatment with higher survival rates and lower toxicities. Adoptive cellular therapy (ACT) has shown promising results in inducing sustainable remissions in patients suffering from refractory diseases. Two main types of ACT include engineered Chimeric Antigen Receptor (CAR) T cells and T cell receptor (TCR) T cells. The application of these immuno-therapies in the last few years has been successful and has demonstrated a safe and rapid treatment regimen for solid and non-solid tumors. The current review presents an insight into the clinical pharmacology aspects of immuno-therapies, especially CAR-T cells. Here, we summarize the current knowledge of TCR and CAR-T cell immunotherapy with particular focus on the structure of CAR-T cells, the effects and toxicities associated with these therapies in clinical trials, risk mitigation strategies, dose selection approaches, and cellular kinetics. Finally, the quantitative approaches and modeling techniques used in the development of CAR-T cell therapies are described.
© 2022 Amgen, Inc. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Johannes Kast, Di Zhou, Marc R. Yago, Po‐Wei Chen, Malidi Ahamadi, Sandeep Dutta, and Vijay V. Upreti are employees of and stockholders in Amgen Inc. Saeideh Nozohouri is a previous employee of Amgen Inc.
Figures
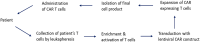

References
-
- Cullen SP, Martin SJ. Mechanisms of granule‐dependent killing. Cell Death Differ. 2008;15:251‐262. - PubMed
-
- de Saint Basile G, Menasche G, Fischer A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat Rev Immunol. 2010;10:568‐579. - PubMed
-
- Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 2020;21:e168‐e178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

