Exposure of Fexofenadine, but Not Pseudoephedrine, Is Markedly Decreased by Green Tea Extract in Healthy Volunteers
- PMID: 35678032
- PMCID: PMC9540489
- DOI: 10.1002/cpt.2682
Exposure of Fexofenadine, but Not Pseudoephedrine, Is Markedly Decreased by Green Tea Extract in Healthy Volunteers
Abstract
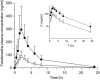
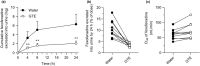
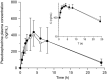
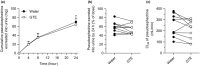
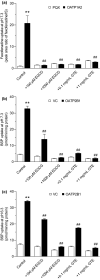
Green tea (GT) alters the disposition of a number of drugs, such as nadolol and lisinopril. However, it is unknown whether GT affects disposition of hydrophilic anti-allergic drugs. The purpose of this study was to investigate whether pharmacokinetics of fexofenadine and pseudoephedrine are affected by catechins, major GT components. A randomized, open, 2-phase crossover study was conducted in 10 healthy Japanese volunteers. After overnight fasting, subjects were simultaneously administered fexofenadine (60 mg) and pseudoephedrine (120 mg) with an aqueous solution of green tea extract (GTE) containing (-)-epigallocatechin gallate (EGCG) of ~ 300 mg or water (control). In vitro transport assays were performed using HEK293 cells stably expressing organic anion transporting polypeptide (OATP)1A2 to evaluate the inhibitory effect of EGCG on OATP1A2-mediated fexofenadine transport. In the GTE phase, the area under the plasma concentration-time curve and the amount excreted unchanged into urine for 24 hours of fexofenadine were significantly decreased by 70% (P < 0.001) and 67% (P < 0.001), respectively, compared with control. There were no differences in time to maximum plasma concentration and the elimination half-life of fexofenadine between phases. Fexofenadine was confirmed to be a substrate of OATP1A2, and EGCG (100 and 1,000 μM) and GTE (0.1 and 1 mg/mL) inhibited OATP1A2-mediated uptake of fexofenadine. On the contrary, the concomitant administration of GTE did not influence the pharmacokinetics of pseudoephedrine. These results suggest that intake of GT may result in a markedly reduced exposure of fexofenadine, but not of pseudoephedrine, putatively by inhibiting OATP1A2-mediated intestinal absorption.
© 2022 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
Similar articles
-
Role of (-)-epigallocatechin gallate in the pharmacokinetic interaction between nadolol and green tea in healthy volunteers.Eur J Clin Pharmacol. 2018 Jun;74(6):775-783. doi: 10.1007/s00228-018-2436-2. Epub 2018 Feb 26. Eur J Clin Pharmacol. 2018. PMID: 29480324 Clinical Trial.
-
Effects of green tea extract and (-)-epigallocatechin-3-gallate on pharmacokinetics of nadolol in rats.Phytomedicine. 2013 Nov 15;20(14):1247-50. doi: 10.1016/j.phymed.2013.07.003. Epub 2013 Aug 3. Phytomedicine. 2013. PMID: 23920278
-
Impact of Green Tea Catechin Ingestion on the Pharmacokinetics of Lisinopril in Healthy Volunteers.Clin Transl Sci. 2021 Mar;14(2):476-480. doi: 10.1111/cts.12905. Epub 2020 Oct 22. Clin Transl Sci. 2021. PMID: 33048477 Free PMC article. Clinical Trial.
-
Green Tea Catechins as Perpetrators of Drug Pharmacokinetic Interactions.Clin Pharmacol Ther. 2025 Jul;118(1):45-61. doi: 10.1002/cpt.3547. Epub 2025 Jan 2. Clin Pharmacol Ther. 2025. PMID: 39748104 Review.
-
An Appraisal of Drug-Drug Interactions with Green Tea (Camellia sinensis).Planta Med. 2017 Apr;83(6):496-508. doi: 10.1055/s-0043-100934. Epub 2017 Jan 24. Planta Med. 2017. PMID: 28118673 Review.
Cited by
-
Developing a Knowledge Graph for Pharmacokinetic Natural Product-Drug Interactions.J Biomed Inform. 2023 Apr;140:104341. doi: 10.1016/j.jbi.2023.104341. Epub 2023 Mar 17. J Biomed Inform. 2023. PMID: 36933632 Free PMC article.
-
Co-consuming green tea with raloxifene decreases raloxifene systemic exposure in healthy adult participants.Clin Transl Sci. 2023 Oct;16(10):1779-1790. doi: 10.1111/cts.13578. Epub 2023 Aug 28. Clin Transl Sci. 2023. PMID: 37639334 Free PMC article.
-
Genomewide association analysis on green tea chemoprevention of colorectal adenoma: the importance of SLCO1A2 variants.Pharmacogenomics. 2025 Apr-Apr;26(5-6):157-164. doi: 10.1080/14622416.2025.2510186. Epub 2025 May 28. Pharmacogenomics. 2025. PMID: 40433816 Clinical Trial.
-
Membrane transporters in drug development and as determinants of precision medicine.Nat Rev Drug Discov. 2024 Apr;23(4):255-280. doi: 10.1038/s41573-023-00877-1. Epub 2024 Jan 24. Nat Rev Drug Discov. 2024. PMID: 38267543 Free PMC article. Review.
-
Cardioprotective and Anti-Hypertensive Effects of Epigallocatechin Gallate: Novel Insights Into Biological Evidence.J Clin Hypertens (Greenwich). 2025 Jun;27(6):e70036. doi: 10.1111/jch.70036. J Clin Hypertens (Greenwich). 2025. PMID: 40495059 Free PMC article. Review.
References
-
- Misaka, S. et al. Green tea ingestion greatly reduces plasma concentrations of nadolol in healthy subjects. Clin. Pharmacol. Ther. 95, 432–438 (2014). - PubMed
-
- Abe, O. et al. Role of (−)‐epigallocatechin gallate in the pharmacokinetic interaction between nadolol and green tea in healthy volunteers. Eur. J. Clin. Pharmacol. 74, 775–783 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

