Förster Resonance Energy Transfer in Luminescent Solar Concentrators
- PMID: 35678107
- PMCID: PMC9376834
- DOI: 10.1002/advs.202201160
Förster Resonance Energy Transfer in Luminescent Solar Concentrators
Abstract
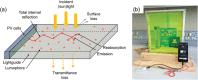
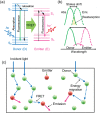
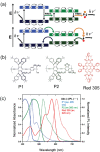
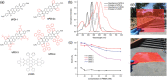
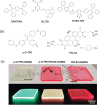
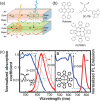
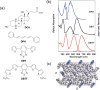
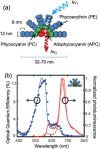
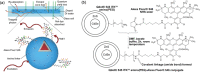
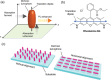
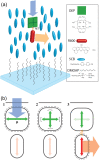
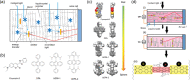
Luminescent solar concentrators (LSCs) are an emerging technology to collect and channel light from a large absorption area into a smaller one. They are a complementary technology for traditional solar photovoltaics (PV), particularly suitable for application in urban or indoor environments where their custom colors and form factors, and performance under diffuse light conditions may be advantageous. Förster resonance energy transfer (FRET) has emerged as a valuable approach to overcome some of the intrinsic limitations of conventional single lumophore LSCs, such as reabsorption or reduced quantum efficiency. This review outlines the potential of FRET to boost LSC performance, using highlights from the literature to illustrate the key criteria that must be considered when designing an FRET-LSC, including both the photophysical requirements of the FRET lumophores and their interaction with the host material. Based on these criteria, a list of design guidelines intended to aid researchers when they approach the design of a new FRET-LSC system is presented. By highlighting the unanswered questions in this field, the authors aim to demonstrate the potential of FRET-LSCs for both conventional solar-harvesting and emerging LSC-inspired technologies and hope to encourage participation from a diverse researcher base to address this exciting challenge.
Keywords: Förster resonance energy transfer; light harvesting; luminescent solar concentrator; lumophore; solar energy.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Renewable Capacity Highlights, IRENA 2020.
-
- Meinardi F., Bruni F., Brovelli S., Nat. Rev. Mater. 2017, 2, 17072.
-
- Rafiee M., Chandra S., Ahmed H., McCormack S. J., Opt. Mater. 2019, 91, 212.
-
- Li Y., Zhang X., Zhang Y., Dong R., Luscombe C. K., J. Polym. Sci., Part A: Polym. Chem. 2019, 57, 201.
-
- Weber W. H., Lambe J., Appl. Opt. 1976, 15, 2299. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
