Oral and Inhaled Fosamprenavir Reverses Pepsin-Induced Damage in a Laryngopharyngeal Reflux Mouse Model
- PMID: 35678265
- PMCID: PMC9732152
- DOI: 10.1002/lary.30242
Oral and Inhaled Fosamprenavir Reverses Pepsin-Induced Damage in a Laryngopharyngeal Reflux Mouse Model
Abstract
Objective: More than 20% of the US population suffers from laryngopharyngeal reflux. Although dietary/lifestyle modifications and alginates provide benefit to some, there is no gold standard medical therapy. Increasing evidence suggests that pepsin is partly, if not wholly, responsible for damage and inflammation caused by laryngopharyngeal reflux. A treatment specifically targeting pepsin would be amenable to local, inhaled delivery, and could prove effective for endoscopic signs and symptoms associated with nonacid reflux. The aim herein was to identify small molecule inhibitors of pepsin and test their efficacy to prevent pepsin-mediated laryngeal damage in vivo.
Methods: Drug and pepsin binding and inhibition were screened by high-throughput assays and crystallography. A mouse model of laryngopharyngeal reflux (mechanical laryngeal injury once weekly for 2 weeks and pH 7 solvent/pepsin instillation 3 days/week for 4 weeks) was provided inhibitor by gavage or aerosol (fosamprenavir or darunavir; 5 days/week for 4 weeks; n = 3). Larynges were collected for histopathologic analysis.
Results: HIV protease inhibitors amprenavir, ritonavir, saquinavir, and darunavir bound and inhibited pepsin with IC50 in the low micromolar range. Gavage and aerosol fosamprenavir prevented pepsin-mediated laryngeal damage (i.e., reactive epithelia, increased intraepithelial inflammatory cells, and cell apoptosis). Darunavir gavage elicited mild reactivity and no discernable protection; aerosol protected against apoptosis.
Conclusions: Fosamprenavir and darunavir, FDA-approved therapies for HIV/AIDS, bind and inhibit pepsin, abrogating pepsin-mediated laryngeal damage in a laryngopharyngeal reflux mouse model. These drugs target a foreign virus, making them ideal to repurpose. Reformulation for local inhaled delivery could further improve outcomes and limit side effects.
Level of evidence: NA. Laryngoscope, 133:S1-S11, 2023.
Keywords: LPR; Laryngopharyngeal reflux; darunavir; fosamprenavir; pepsin.
© 2022 The American Laryngological, Rhinological and Otological Society, Inc.
Conflict of interest statement
Figures
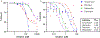

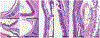

References
-
- Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope 1991;101:1–78. - PubMed
-
- Vaezi MF. Extraesophageal manifestations of gastroesophageal reflux disease. Clin Cornerstone 2003;5:32–38; discussion 39–40. - PubMed
-
- Ford CN. Evaluation and management of laryngopharyngeal reflux. JAMA 2005;294:1534–1540. - PubMed
-
- Bianchi ET, Guerreiro Cardoso PF, Minamoto H, et al. Impact of fundoplication for gastroesophageal reflux in the outcome of benign tracheal stenosis. J Thorac Cardiovasc Surg 2019;158:1698–1706. - PubMed
-
- Esposito C, Saxena A, Irtan S, Till H, Escolino M. Laparoscopic Nissen Fundoplication: An Excellent Treatment of GERD-Related Respiratory Symptoms in Children-Results of a Multicentric Study. J Laparoendosc Adv Surg Tech A 2018;28:1023–1028. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

