Optimal dosing of cefotaxime and desacetylcefotaxime for critically ill paediatric patients. Can we use microsampling?
- PMID: 35678266
- PMCID: PMC9333413
- DOI: 10.1093/jac/dkac168
Optimal dosing of cefotaxime and desacetylcefotaxime for critically ill paediatric patients. Can we use microsampling?
Abstract
Objectives: To describe the population pharmacokinetics of cefotaxime and desacetylcefotaxime in critically ill paediatric patients and provide dosing recommendations. We also sought to evaluate the use of capillary microsampling to facilitate data-rich blood sampling.
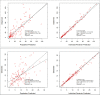
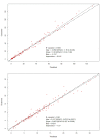
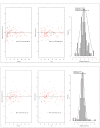
Methods: Patients were recruited into a pharmacokinetic study, with cefotaxime and desacetylcefotaxime concentrations from plasma samples collected at 0, 0.5, 2, 4 and 6 h used to develop a population pharmacokinetic model using Pmetrics. Monte Carlo dosing simulations were tested using a range of estimated glomerular filtration rates (60, 100, 170 and 200 mL/min/1.73 m2) and body weights (4, 10, 15, 20 and 40 kg) to achieve pharmacokinetic/pharmacodynamic (PK/PD) targets, including 100% ƒT>MIC with an MIC breakpoint of 1 mg/L.
Results: Thirty-six patients (0.2-12 years) provided 160 conventional samples for inclusion in the model. The pharmacokinetics of cefotaxime and desacetylcefotaxime were best described using one-compartmental model with first-order elimination. The clearance and volume of distribution for cefotaxime were 12.8 L/h and 39.4 L, respectively. The clearance for desacetylcefotaxime was 10.5 L/h. Standard dosing of 50 mg/kg q6h was only able to achieve the PK/PD target of 100% ƒT>MIC in patients >10 kg and with impaired renal function or patients of 40 kg with normal renal function.
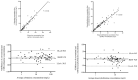
Conclusions: Dosing recommendations support the use of extended or continuous infusion to achieve cefotaxime exposure suitable for bacterial killing in critically ill paediatric patients, including those with severe or deep-seated infection. An external validation of capillary microsampling demonstrated skin-prick sampling can facilitate data-rich pharmacokinetic studies.
© The Author(s) 2022. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures

References
-
- Schlapbach LJ, Straney L, Alexander Jet al. . Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand 2002-13: a multicentre retrospective cohort study. Lancet Infect Dis 2015; 15: 46–54. - PubMed
-
- Jablensky A, Johnson R, Bunney Wet al. . Neurological, Psychiatric, and Developmental Disorders; Meeting the Challenge in the Developing World. National Academy Press, 2001. - PubMed
-
- Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 2009; 37: 840–51. - PubMed
-
- Osowicki J, Gwee A, Noronha Jet al. . Australia-wide point prevalence survey of antimicrobial prescribing in neonatal units: how much and how good? Pediatr Infect Dis J 2015; 34: e185–90. - PubMed
-
- Shein SL, Kong M, McKee Bet al. . Antibiotic prescription in young children with respiratory syncytial virus-associated respiratory failure and associated outcomes. Pediatr Crit Care Med 2019; 20: 101–9. - PubMed

