Alpha7 nicotinic acetylcholine receptor mediates chronic nicotine inhalation-induced cardiopulmonary dysfunction
- PMID: 35678315
- PMCID: PMC10199464
- DOI: 10.1042/CS20220083
Alpha7 nicotinic acetylcholine receptor mediates chronic nicotine inhalation-induced cardiopulmonary dysfunction
Abstract
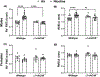
Cigarette smoking remains the leading modifiable risk factor for cardiopulmonary diseases; however, the effects of nicotine alone on cardiopulmonary function remain largely unknown. Previously, we have shown that chronic nicotine vapor inhalation in mice leads to the development of pulmonary hypertension (PH) with right ventricular (RV) remodeling. The present study aims to further examine the cardiopulmonary effects of nicotine and the role of the α7 nicotinic acetylcholine receptor (α7-nAChR), which is widely expressed in the cardiovascular system. Wild-type (WT) and α7-nAChR knockout (α7-nAChR-/-) mice were exposed to room air (control) or nicotine vapor daily for 12 weeks. Consistent with our previous study, echocardiography and RV catheterization reveal that male WT mice developed increased RV systolic pressure with RV hypertrophy and dilatation following 12-week nicotine vapor exposure; in contrast, these changes were not observed in male α7-nAChR-/- mice. In addition, chronic nicotine inhalation failed to induce PH and RV remodeling in female mice regardless of genotype. The effects of nicotine on the vasculature were further examined in male mice. Our results show that chronic nicotine inhalation led to impaired acetylcholine-mediated vasodilatory response in both thoracic aortas and pulmonary arteries, and these effects were accompanied by altered endothelial nitric oxide synthase phosphorylation (enhanced inhibitory phosphorylation at threonine 495) and reduced plasma nitrite levels in WT but not α7-nAChR-/- mice. Finally, RNA sequencing revealed up-regulation of multiple inflammatory pathways in thoracic aortas from WT but not α7-nAChR-/- mice. We conclude that the α7-nAChR mediates chronic nicotine inhalation-induced PH, RV remodeling and vascular dysfunction.
Keywords: alpha7-nAChR; eNOS/NO signaling; endothelial dysfunction; nicotine; pulmonary hypertension; right ventricular remodeling.
© 2022 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Figures

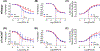
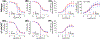


Similar articles
-
α7 Nicotinic acetylcholine receptor mediates right ventricular fibrosis and diastolic dysfunction in pulmonary hypertension.JCI Insight. 2021 Jun 22;6(12):e142945. doi: 10.1172/jci.insight.142945. JCI Insight. 2021. PMID: 33974567 Free PMC article.
-
Effects of Chronic Nicotine Inhalation on Systemic and Pulmonary Blood Pressure and Right Ventricular Remodeling in Mice.Hypertension. 2020 May;75(5):1305-1314. doi: 10.1161/HYPERTENSIONAHA.119.14608. Epub 2020 Mar 16. Hypertension. 2020. PMID: 32172623 Free PMC article.
-
Effect of α7 nicotinic acetylcholine receptor activation on cardiac fibroblasts: a mechanism underlying RV fibrosis associated with cigarette smoke exposure.Am J Physiol Lung Cell Mol Physiol. 2017 May 1;312(5):L748-L759. doi: 10.1152/ajplung.00393.2016. Epub 2017 Mar 3. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28258105 Free PMC article.
-
Angiotensin II type 1 receptor mediates pulmonary hypertension and right ventricular remodeling induced by inhaled nicotine.Am J Physiol Heart Circ Physiol. 2021 Apr 1;320(4):H1526-H1534. doi: 10.1152/ajpheart.00883.2020. Epub 2021 Feb 12. Am J Physiol Heart Circ Physiol. 2021. PMID: 33577434 Free PMC article.
-
Nicotine elevated intracellular Ca²⁺ in rat airway smooth muscle cells via activating and up-regulating α7-nicotinic acetylcholine receptor.Cell Physiol Biochem. 2014;33(2):389-401. doi: 10.1159/000356678. Epub 2014 Feb 6. Cell Physiol Biochem. 2014. PMID: 24525957
Cited by
-
Nicotinic receptors in airway disease.Am J Physiol Lung Cell Mol Physiol. 2024 Feb 1;326(2):L149-L163. doi: 10.1152/ajplung.00268.2023. Epub 2023 Dec 12. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38084408 Free PMC article. Review.
-
Ovarian hormones do not mediate protection against pulmonary hypertension and right ventricular remodeling in female mice exposed to chronic, inhaled nicotine.Am J Physiol Heart Circ Physiol. 2022 Nov 1;323(5):H941-H948. doi: 10.1152/ajpheart.00467.2022. Epub 2022 Oct 7. Am J Physiol Heart Circ Physiol. 2022. PMID: 36206053 Free PMC article.
-
Nicotine and novel tobacco products drive adverse cardiac remodeling and dysfunction in preclinical studies.Front Cardiovasc Med. 2022 Oct 6;9:993617. doi: 10.3389/fcvm.2022.993617. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36277777 Free PMC article.
-
Cardiovascular dysfunction induced by combined exposure to nicotine inhalation and high-fat diet.Am J Physiol Heart Circ Physiol. 2024 Jan 1;326(1):H278-H290. doi: 10.1152/ajpheart.00474.2023. Epub 2023 Dec 1. Am J Physiol Heart Circ Physiol. 2024. PMID: 38038717 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

