Towards a unified model of naive T cell dynamics across the lifespan
- PMID: 35678373
- PMCID: PMC9348855
- DOI: 10.7554/eLife.78168
Towards a unified model of naive T cell dynamics across the lifespan
Abstract
Naive CD4 and CD8 T cells are cornerstones of adaptive immunity, but the dynamics of their establishment early in life and how their kinetics change as they mature following release from the thymus are poorly understood. Further, due to the diverse signals implicated in naive T cell survival, it has been a long-held and conceptually attractive view that they are sustained by active homeostatic control as thymic activity wanes. Here we use multiple modelling and experimental approaches to identify a unified model of naive CD4 and CD8 T cell population dynamics in mice, across their lifespan. We infer that both subsets divide rarely, and progressively increase their survival capacity with cell age. Strikingly, this simple model is able to describe naive CD4 T cell dynamics throughout life. In contrast, we find that newly generated naive CD8 T cells are lost more rapidly during the first 3-4 weeks of life, likely due to increased recruitment into memory. We find no evidence for elevated division rates in neonates, or for feedback regulation of naive T cell numbers at any age. We show how confronting mathematical models with diverse datasets can reveal a quantitative and remarkably simple picture of naive T cell dynamics in mice from birth into old age.
Keywords: computational biology; immunology; inflammation; mathematical modeling; mouse; naive T cells; population dynamics; systems biology.
© 2022, Rane, Hogan et al.
Conflict of interest statement
SR, TH, EL, BS, AY No competing interests declared
Figures

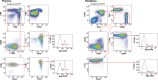


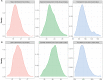

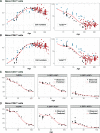

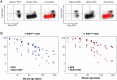
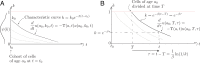
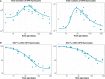
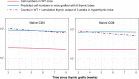
Comment in
- doi: 10.7554/eLife.81077
References
-
- Akaike H. On the likelihood of a time series model. The Statistician. 1978;27:217. doi: 10.2307/2988185. - DOI
-
- Baliu-Piqué M, van Hoeven V, Drylewicz J, van der Wagen LE, Janssen A, Otto SA, van Zelm MC, de Boer RJ, Kuball J, Borghans JA, Tesselaar K. Cell-density independent increased lymphocyte production and loss rates post-autologous HSCT. eLife. 2021;10:e59775. doi: 10.7554/eLife.59775. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

